Abstract
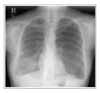
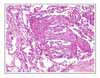
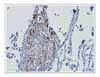
Lymphangioleiomyomatosis (LAM) is a rare disease that affects females of reproductive age. It is characterized by the abnormal proliferation of smooth muscle cells in the lung and along the axial lymphatics. We report a case of lymphangioleiomyomatosis presenting as a lung mass. The patient visited the emergency room because of dyspnea upon exertion. The chest X-ray showed a lung mass in the right lower lung field and a pneumothorax in the left lung. Chest computed tomography revealed a 5 × 3 cm sized mass in the right lower lobe and multiple thin-walled small cysts scattered in both lungs. Transbronchial biopsy of the lung mass was performed. The biopsy specimen showed atypical smooth muscle cell proliferation and cystic dilatation of the terminal bronchioles, which confirmed the diagnosis of lymphangioleiomyomatosis. To the best of our knowledge, this is the first case of lymphangioleiomyomatosis presenting as a lung mass.
Figures and Tables
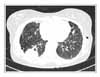
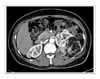
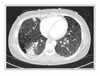
Figure 2
Chest computed tomography shows a 5 × 3 cm-sized mass in right lower lobe and multiple thin-walled small cysts scattered in both lungs.
References
1. Johnson SR. lymphangioleiomyomatosis. Eur Respir J. 2006. 27:1056–1065.
2. Oh YM, Mo EK, Jang SH, Yoo CG, Kim YW, Seo JW, et al. Pulmonary lymphangioleiomyomatosis in Korea. Thorax. 1999. 54:618–621.
3. Kenerson HL, Aicher LD, True LD, Yeung RS. Activated mammalian target of rapamycin pathway in the pathogenesis of tuberous sclerosis complex renal tumors. Cancer Res. 2002. 62:5645–5650.
4. Matsumoto Y, Horiba K, Usuki J, Chu SC, Ferrans VJ, Moss J. Markers of cell proliferation and expression of melanosomal antigen in lymphangioleiomyomatosis. Am J Respir Cell Mol Biol. 1999. 21:327–336.
5. Banner AS. Hormone receptors in lymphangioleiomyomatosis. Chest. 1984. 85:3–4.
6. Matsui K, Takeda K, Yu ZX, Travis WD, Moss J, Ferrans VJ. Role for activation of matrix metalloproteinases in the pathogenesis of pulmonary lymphangioleiomyomatosis. Arch Pathol Lab Med. 2000. 124:267–275.
7. Johnson SR, Tattersfield AE. Decline in lung function in lymphangioleiomyomatosis: relation to menopause and progesterone treatment. Am J Respir Crit Care Med. 1999. 160:628–633.
8. Taveira-DaSilva AM, Stylianou MP, Hedin CJ, Hathaway O, Moss J. Decline in lung function in patients with lymphangioleiomyomatosis treated with or without progesterone. Chest. 2004. 126:1867–1874.
9. Taylor JR, Ryu J, Colby TV, Raffin TA. Lymphangioleiomyomatosis. Clinical course in 32 patients. N Engl J Med. 1990. 323:1254–1260.
10. Urban T, Lazor R, Lacronique J, Murris M, Labrune S, Valeyre D, et al. Groupe d'Etudes et de Recherche sur les Maladies "Orphelines" Pulmonaires (GERM"O"P). Pulmonary lymphangioleiomyomatosis. A study of 69 patients. Medicine (Baltimore). 1999. 78:321–337.
11. Johnson SR, Whale CI, Hubbard RB, Lewis SA, Tattersfield AE. Survival and disease progression in UK patients with lymphangioleiomyomatosis. Thorax. 2004. 59:800–803.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download