Acinetobacter baumannii, an aerobic Gram-negative coccobacillus, is ubiquitous in fresh water and soil. As a frequent skin and oropharyngeal commensal, it is also a well-recognized as nosocomial pathogen, particularly in pneumonia following endotracheal intubation, prolonged mechanical ventilation, underlying lung diseases, prior broad-spectrum antibiotic treatment, enteric feeding, or who are being treated in an intensive care unit1,2.
A 70 year-old-man was admitted to the hospital because of right-sided chest pain, fever, and cough productive of yellow sputum for 7 days. He denied weight loss, night sweats, or hemoptysis. He had a history of hypertension and 13-pack-year history of cigarette smoking. He was a retired man and drank moderate amounts of alcohol. There was no history of diabetes, aspiration, and recent travel.
Clinical examination revealed a slightly tachypneic patient with blood pressure of 140/80 mm Hg, temperature of 37.8℃, heart rate of 83 beats/min, and respiratory rate of 27 breaths/min. The lung examination revealed scattered rhonchi and reduced breath sounds over the right lung base. The findings of the remainder of the examination were unremarkable. Pulmonary function tests showed FVC of 2.72 L (69% of predicted); FEV1 of 1.75 L (66% of predicted); FEV1/FVC ratio of 64%, suggesting moderate airway obstruction due to COPD. Arterial blood gas analysis showed pH of 7.44; PaCO2, 35.7 mm Hg; PaO2, 70.1 mm Hg; and oxygen saturation, 94.5% while he was breathing ambient air.
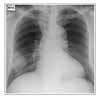
The initial results of laboratory tests, liver function tests, renal function test, and urinalysis were all within normal limits. WBC count was 10,400/mm3 with 79% neutrophils, 12% lymphocytes, and 7% monocytes. The serum C-reactive protein level was 7.87 mg/dl, and the erythrocyte sedimentation rate was 79 mm/h. The hemoglobin level was 12.6 g/dl, and the platelet count was 225,000/mm3. A chest radiograph obtained at presentation showed air space opacity at the right lower lobe (Figure 1). A presumed diagnosis of community acquired pneumonia was made.
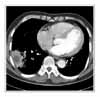
Appropriate blood and sputum cultures were obtained, and antibiotic therapy was started on cefotaxime 2.0 g IV q8 hr plus amikacin 250 mg IV q12 hr and roxithromycin 150 mg bid. Gram staining of a sputum specimen revealed many polymorphonuclear leukocytes, few epithelial cells, and many gram negative cocci. Chest CT scan revealed air space consolidation with internal low-attenuation area, and surrounding infiltrates in the right lower lung, a finding consistent with necrotizing pneumonia (Figure 2).
Fever disappeared during the following 48 hours but chest radiography revealed sustained consolidation. On the 6th day, amikacin was discontinued and clindamycin begun to cover for possible anaerobe. On the 7th day, the first sputum cultures yielded a growth of A. baumannii; the organism was sensitive to all tested multiple antibiotics, including amikacin, ceftriaxone, ceftazidime, gentamicin, levofloxacin, and ciprofloxacin. Cefotaxime was not included in the susceptibility test. However, our patient continued to receive cefotaxime based on patient's clinical response. Blood cultures were negative for A. baumannii. Sputum acid-fast bacilli smears were also negative and culture results were pending. Laboratory evaluation for Legionella and Mycoplasma were negative. On the 10th days, Second sputum culture also yielded a growth of A. baumannii to which all tested antibiotics were susceptible.
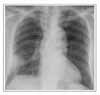
On the 11th hospital day, needle aspiration biopsy for a sustained lesion on chest radiography was performed. Aspirates Gram stain and subsequent cultures were negative. The histology of biopsy specimen showed the replacement of lung parenchyma by chronic inflammation and fibrosis. Bronchoscopy was not performed. After treatment with antibiotics, chest pain and productive cough diminished and the patient's condition was improved. Chest X-ray also showed some improvement (Figure 3).
On the 19th hospital day, he was discharged while taking amoxicillin/clavulanate for 5 weeks, and then switched to moxifloxacin for an additional 4 weeks. At the 12-week follow-up, the patient reported complete resolution of symptoms. A repeat chest radiograph showed near-complete resolution of the right lower lobe infiltrate.
Although A. baumannii is not a well-recognized pathogen causing community acquired pneumonia, its importance increases in tropical and humid countries, as shown by its identification in 10% of all community-acquired bacteremic pneumonia and 21% of Gram-negative pneumonia in northern Australia3.
Community-acquired Acinetobacter pneumonia generally occurs in patients with diminished host defenses such as alcoholics (50%), cigarette smoking (48%), COPD (34%), and diabetes (16%)4. The incidence of pneumonia in alcoholics was reported to be 31%7 to 46%4. Cigarette smoking, COPD, and alcoholics among recognized risk factors were present in our patient.
Due to the wide distribution in nature and the ability to colonize healthy or damaged tissue, interpreting the significance of isolates from clinical specimens of Acinetobacter species is often difficult. Additionally, Acinetobacter species is often misinterpreted as Gram-positive cocci owing to its tendency to retain crystal violet on Gram's staining8.
The isolation of A. baumannii from sputum may occur in 69% of patients7. A good sputum smear, defined as a Gram stain smear of an adequate sputum specimen that comes from the lower respiratory tract and contains > 25 leukocytes per high-power field (100 ×) on microscopic examination, may help the initial diagnosis. Blood cultures were positive in 28 of 34 cases3.
Patients with community-acquired Acinetobacter pneumonia often present with the acute onset of severe respiratory distress, tachypnea, fever, productive cough, and pleuritic pain7. Shock often develops within 24 hours of hospital admission. The chest radiography may reveal either lobar consolidation or bronchopneumonia, but progression to diffuse and bilateral involvement often occurs rapidly9. Rarely, an empyema or an abscess and multiple cavities may complicate the initial infection. Our case had an extensive necrotizing pneumonia but resolved slowly after therapy.
Current antibiotic guidelines are not targeted toward treating A. baumannii because of uncommon pathogen in CAP10,11. Therefore, it is very important that we have increased awareness of A. baumannii as a cause of severe CAP. The major problem related to these microorganisms is multiple drug resistance that may lead to therapeutic problems13. But strains of A. baumannii causing community-acquired infections are usually susceptible to aminoglycosides, extended-spectrum penicillins, ceftazidime, quinolone, and imipenem3,4. Treatment with a combination of an aminoglycoside and antipseudomonal penicillin or a carbapenem is usually recommended for pneumonia caused by Acinetobacter species3. Presented case responded well to early treatment with antibiotics to which A. baumannii is susceptible.
Mortality rate is high (40-64%)4,6 and comparable to those reported for patients with severe CAP due to S. pneumoniae (40 to 75%), Legionella pneumophilia (33 to 56%), Staphylococcus aureus (72 to 100%) in the Nottingham14. High risk factors for higher mortality were bacteremia, low platelet count (<120 × 109 cells/L), pH <7.35 on presentation, and disseminated intravascular coagulation (DIC)12.
This case doesn't provide a definite evidence of A. baumannii recovered from blood or tissue but suggestive of A. baumannii community acquired pneumonia. Isolation of A. baumannii from sputum in otherwise healthy person is unusual. Throat carriage of A. baumannii by community subjects was not found in Hong Kong15. In our case, in which we initially diagnosed as community acquired pneumonia with favorable response to the empirical antibiotics, the result that A. baumannii was recovered from sputum without isolation from blood or tissue can't be considered simple colonization or contamination of A. baumannii. Initiation of prompt and appropriate antibiotic treatment subsequently found to have negative culture. Alcohol consumption as in our case is the major risk factor for community acquired A. baumannii pneumonia3, and microaspiration of pharyngeal organisms is postulated to precede community acquired A. baumannii pneumonia in those with alcoholism.
Figures and Tables
Figure 1
A chest radiograph obtained at presentation shows air space opacity at the right lower lobe.
References
1. Kaul R, Burt JA, Cork L, Dedier H, Garcia M, Kennedy C, et al. Investigation of a multiyear multiple critical care unit outbreak due to relative drug-sensitive Acinetobacter baumannii: risk factors and attributable mortality. J Infect Dis. 1996. 174:1279–1289.
2. Forster DH, Daschner FD. Acinetobacter species as nosocomial pathogens. Eur J Clin Microbiol Infect Dis. 1998. 17:73–77.
3. Anstey NM, Currie BJ, Withnall KM. Community-acquired Acinetobacter pneumonia in the northern territory of Australia. Clin Infect Dis. 1992. 14:83–91.
4. Bick JA, Semel JD. Fulminant community-acquired Acinetobacter pneumonia in a healthy woman. Clin Infect Dis. 1993. 17:820–821.
5. Park II, Kim IK, Koo HC, Han JP, Kim YM, Lee MG, et al. Clinical characteristics and prognosis of Acinetobacter nosocomial pneumonia between MDR and non-MDR. Tuberc Respir Dis. 2006. 61:13–19.
6. Lee MS, Lee SO, Kim YS, Hong SS, Choi SY, Park SJ, et al. A prospective study of nosocomial acquisition of Acinetobacter baumannii in a medical intensive care unit. Korean J Infect Dis. 2000. 32:8–20.
7. Chen MZ, Hsueh PR, Lee LN, Yu CJ, Yang PC, Luh KT. Severe community-acquired pneumonia due to Acinetobacter baumannii. Chest. 2001. 120:1072–1077.
8. Goodhart GL, Abrutyn E, Watson R, Root RK, Egert J. Community acquired Acinetobacter calcoaceticus var anitratus pneumonia. JAMA. 1977. 238:1516–1518.
9. Achar KN, Johny M, Achar MN, Menon NK. Community-acquired bacteraemic Acinetobacter pneumonia with survival. Postgrad Med J. 1993. 69:934–937.
10. Mandell LA, Bartlett JG, Dowell SF, File TM Jr, Musher DM, Whitney CG, et al. Update of practice guidelines for the management of community-acquired pneumonia in immunocompetent adults. Clin Infect Dis. 2003. 37:1405–1433.
11. Niederman MS, Mandell LA, Anzueto A, Bass JB, Broughton WA, Campbell GD, et al. Guidelines for the management of adults with community-acquired pneumonia: diagnosis, assessment of severity, antimicrobial therapy, and prevention. Am J Respir Crit Care Med. 2001. 163:1730–1754.
12. Leung WS, Chu CM, Tsang KY, Lo FH, Lo KF, Ho PL. Fulminant community-acquired Acinetobacter baumannii pneumonia as a distinct clinical syndrome. Chest. 2006. 129:102–109.
13. Graser Y, Klare I, Halle E, Graser Y, Gantenberg R, Buchholz P, et al. Epidemiological study of an Acinetobacter baumannii outbreak by using polymerase chain reaction fingerprinting. J Clin Microbiol. 1993. 31:2417–2420.
14. Hirani NA, Macfarlane JT. Impact of management guidelines on the outcome of severe community acquired pneumonia. Thorax. 1997. 52:17–21.
15. Chu YW, Leung CM, Houang ET, Ng KC, Leung CB, Leung HY, et al. Skin carriage of Acinetobacters in Hong Kong. J Clin Microbiol. 1999. 37:2962–2967.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download