Abstract
Background
Asthma is a syndrome that is characterized by a variable degree of airflow obstruction, bronchial hyperresponsiveness, and airway inflammation. Colchicine is an inexpensive and safe medication with unique anti-inflammatory properties. IL-1Ra (Interleukin-1 receptor antagonist) mediates the anti-inflammatory effect in human inflammatory diseases, including asthma. This study examined whether IL-1Ra mediates the anti-inflammatory effect of colchicine in normal human bronchial epithelial cells (NHBE), RAW 264.7 cells (murine macrophage cell line), and a mouse lung.
Methods
NHBE, RAW 264.7 cells and BALB/c mice were stimulated with colchicine, and the increase in the IL-1Ra level was estimated by ELISA, Western analysis and RT-PCR analysis.
Results
Colchicine stimulated NHBE and RAW 264.7 cells to release IL-1Ra into the supernatant in a dose-and time-dependent manner. The major isoform of IL-1Ra in NHBE and RAW 264.7 cells is type I icIL-1Ra, and sIL-1Ra, respectively. IL-1Ra up-regulation was blocked by PD98059, a specific inhibitor in MAPK pathways. Colchicine also stimulated the secretion of IL-1Ra into the bronchoalveolar lavage (BAL) fluid of BALB/c mouse.
Figures and Tables
 | Figure 1Dose kinetics of IL-1Ra elaboration in colchicine treated NHBE. (A) NHBE were incubated in various colchicine concentration media for 48 hours. The levels of immunoreactive IL-1Ra in supernatants were quantitated by ELISA. Colchicine stimulates NHBE to release IL-1Ra into supernatant with dose dependent manner. IL-1Ra elaborations in 0.1 and 0.5 µM colchicine concentrations show statistically significant differences compared to control (*p<0.05). However, in high colchicine concentrations, IL-1Ra elaborations are decreased. The noted values represent the mean ± SEM of a minimum of five assays. (B) Western blot results also show dose dependent IL-1Ra elaboration. SEM: standard error of the mean. |
 | Figure 2Time kinetics of IL-1Ra elaboration in colchicine treated NHBE. (A) NHBE were incubated in 0.1 µM colchicine concentration media for 2, 4, 8, 16, 24, 48 hours. The levels of immunoreactive IL-1Ra in supernatants were quantitated by ELISA. Colchicine stimulates NHBE to release IL-1Ra into supernatant with time dependent manner. IL-1Ra elaboration shows statistically significant differences compared to control after 16 hours (*p<0.05). The noted values represent the mean ± SEM of a minimum of five assays. (B) Western blot results show also time dependent IL-1Ra elaboration. |
 | Figure 3IL-1Ra isoform mRNA expression in colchicine treated NHBE. NHBE were incubated in various colchicine concentration media for 48 hours. The supernatant was removed, total cellular RNA was isolated, and RT-PCR was performed with primers specific for icIL-1Ra type I, icIL-1Ra type II, and β actin. icIL-1Ra I mRNA expressions are dominantly observed. However, there are no differences in IL-1Ra mRNA expression in various colchicine concentrations. |
 | Figure 4IL-1Ra elaboration in NHBE treated with colchicine variants. NHBE were incubated in media containing colchicine variants for 16 hours. The levels of immunoreactive IL-1Ra in supernatants were quantitated by ELISA. Colchicine variants do not stimulate IL-1Ra elaboration whereas chochicine does. The noted values represent the mean + SEM of a minimum of five assays. |
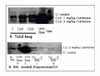 | Figure 5IL-1Ra elaboration in colchicine treated mouse lung. Colchicine was administrated intraperitoneally to BALB/c mouse. The mouse was sacrificed 48 hours later, the elaborations of IL-1Ra in the total lung and bronchoalveolar lavage (BAL) fluid were quantitated by Western blot. (A) Colchicine stimulates to release IL-1Ra with dose dependent manner in mouse lung. (B) Clochicine stimulates to release IL-1Ra in BAL fluid. The lysate shows more dominant band than supernatant. rIL-1Ra (recombinant human nonglycosylated mature sIL-1Ra) and LPS treated RAW 264.7 cells (LPS Raw; 100 ng/ml for 48 hours) are used as positive contols for IL-1Ra elaboration. |
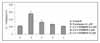 | Figure 6Effect of PD98059 on IL-1Ra elaboration in colchicine treated NHBE. A. NHBE were incubated in various colchicine concentration media for 16 hours. The levels of immunoreactive IL-1Ra in supernatant were quantitated by ELISA. IL-1Ra elaboration by colchicine stimulation is inhibited by MAPK inhibitor (PD98059) with dose dependent manner. The noted values represent the mean + SEM of a minimum of five assays. |
 | Figure 7IL1-Ra isoform mRNA expression in colchicine/PD98059 treated RAW 264.7 cells. RAW 264.7 cells were incubated in various colchicine/PD98059 concentration media for 48 hours. The supernatant was removed, total cellular RNA was isolated, and RT-PCR was performed with primers specific for sIL-1Ra, icIL-1Ra type I. sIL-1Ra mRNA expression is dominantly observed in RAW 264.7 cell than icIL-1Ra type I, and mRNA expression by colchicine is decreased by PD98059 in dose-dependent manner. |
References
1. Busse WW, Lemanske RF Jr. Asthma. N Engl J Med. 2001. 344:350–362.
2. Haley KJ, Sunday ME, Wiggs BR, Kozakewich HP, Reilly JJ, Mentzer SJ, et al. Inflammatory cell distribution within and along asthmatic airways. Am J Respir Crit Care Med. 1998. 158:565–572.
3. Kraft M, Djukanovic R, Wilson S, Holgate ST, Martin RJ. Alveolar tissue inflammation in asthma. Am J Respir Crit Care Med. 1996. 154:1505–1510.
4. Vignola AM, Chanez P, Campbell AM, Souques F, Lebel B, Enander I, et al. Airway inflammation in mild intermittent and in persistent asthma. Am J Respir Crit Care Med. 1998. 157:403–409.
5. Schwarz YA, Kivity S, Ilfeld DN, Schlesinger M, Greif J, Topilsky M, et al. A clinical and immunologic study of colchicines in asthma. J Allergy Clin Immunol. 1990. 85:578–582.
6. Kelly SJ, Uri AJ, Freeland HS, Woods EJ, Schulman ES, Peters SP, et al. Effects of colchicines on IgE-mediated early and late airway reactions. Chest. 1995. 107:985–991.
7. Arend WP, Malyak M, Guthridge CJ, Gabay C. Interleukin-1 receptor antagonist: role in biology. Annu Rev Immunol. 1998. 16:27–55.
8. Selig W, Tocker J. Effect of interleukin-1 receptor antagonist on antigen-induced pulmonary responses in guinea pigs. Eur J Pharmacol. 1992. 213:331–336.
9. Okada S, Inoue H, Yamauchi K, Iijima H, Ohkawara Y, Takishima T, et al. Potential role of interleukin-1 in allergen-induced late asthmatic reactions in guinea pigs: suppressive effect of interleukin-1 receptor antagonist on late asthmatic reaction. J Allergy Clin Immunol. 1995. 95:1236–1245.
10. Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenolchloroform extraction. Anal Biochem. 1987. 162:156–159.
11. Levine SJ, Wu T, Shelhamer JH. Extracellular release of the type I intracellular IL-1 receptor antagonist from human airway epithelial cells: differential effects of IL-4, IL-13, IFN-gamma, and corticosteroids. J Immunol. 1997. 158:5949–5957.
12. Berger AE, Carter DB, Hankey SO, McEwan RN. Cytokine regulation of the interleukin-1 receptor antagonist protein in U937 cells. Eur J Immunol. 1993. 23:39–45.
13. Muzio M, Polentarutti N, Sironi M, Poli G, De Gioia L, Introna M, et al. Cloning and characterization of a new isoform of the interleukin receptor antagonist. J Exp Med. 1995. 182:623–628.
14. Wallace SL, Omokoku B, Ertel NH. Colchicine plasma levels: implications as to pharmacology and mechanism of action. Am J Med. 1970. 48:443–448.
15. Ouyang Y, Wang W, Bhuta S, Chang YH. Mechanism of action of colchicine. VI: Effect of colchicine on generation of leukotriene B4 by human polymorphonuclear leukocytes. Clin Exp Rheumatol. 1989. 7:397–402.
16. Reibman J, Haines KA, Rich AM, Cristello P, Giedd KN, Weissmann G. Colchicine inhibits ionophoreinduced formation of leukotriene B4 by human neutrophils: the role of microtubules. J Immunol. 1986. 136:1027–1032.
17. Griffiths RJ, Li SW, Wood BE, Blackham A. A comparison of the anti-inflammatory activity of selective 5-lipoxygenase inhibitors with dexamethasone and colchicine in a model of zymosan induced inflammation in the rat knee joint and peritoneal cavity. Agents Actions. 1991. 32:312–320.
18. Gillespie E, Lichtenstein LM. Histamine release from human leukocytes: studies with deuterium oxide, colchicine, and cytochalasin B. J Clin Invest. 1972. 51:2941–2947.
19. Kaliner M. Human lung tissue and anaphylaxis. Evidence that cyclic nucleotides modulate the immunologic release of mediators through effects on microtubular assembly. J Clin Invest. 1977. 60:951–959.
20. Rudolph SA, Greengard P, Malawista SE. Effects of colchicines on cyclic AMP levels in human leukocytes. Proc Natl Acad Sci USA. 1977. 74:3404–3408.
21. Hagmann J, Fishman PH. Modulation of adenylate cyclase in intact macrophages by microtubules. Opposing actions of colchicine and chemotactic factor. J Biol Chem. 1980. 255:2659–2662.
22. Danon YL, Zemer D. Low asthma prevalence found in FMF. Immunol Allergy Pract. 1992. 14:357–362.
23. Adalioglu G, Turktas I, Saraclar Y, Tuncer A. A clinical study of colchicines in childhood asthma. J Asthma. 1994. 31:361–366.
24. Newman KB, Mason UG, Buchmeier A, Schmaling KB, Corsello P, Nelson HS. Failure of colchicines to reduce inhaled triamcinolone dose in patients with asthma. J Allergy Clin Immunol. 1997. 99:176–178.
25. Patterson D, Jones C, Hart I, Bleskan J, Berger R, Geyer D, et al. The human interleukin-1 receptor antagonist(IL1RN) gene is located in the chromosome 2q14region. Genomics. 1993. 15:173–176.
26. Malyak M, Smith MF Jr, Abel AA, Hance KR, Arend WP. The differential production of three forms of IL-1 receptor antagonist by human neutrophils and monocytes. J Immunol. 1998. 161:2004–2010.
27. Weissbach L, Tran K, Colquhoun SA, Champliaud MF, Towle CA. Detection of an interleukin-1 intracellular receptor antagonist mRNA variant. Biochem Biophys Res Commun. 1998. 244:91–95.
28. Mulligan MS, Ward PA. Immune complex-induced lung and dermal vascular injury. Differing requirements for tumor necrosis factor-α and IL-1. J Immunol. 1992. 149:331–339.
29. Shanley TP, Peters JL, Jones ML, Chensue SW, Kunkel SL, Ward PA. Regulatory effects of endogenous interleukin-1 receptor antagonist protein in immunoglobulin G immune complex-induced lung injury. J Clin Invest. 1996. 97:963–970.
30. Tillie-Leblond I, Pugin J, Marquette CH, Lamblin C, Saulnier F, Brichet A, et al. Balance between proinflammatory cytokines and their inhibitors in bronchial lavage from patients with status asthmaticus. Am J Respir Crit Care Med. 1999. 159:487–494.
31. Chang L, Karin M. Mammalian MAP kinase signaling cascades. Nature. 2001. 410:37–40.
32. Pelaia G, Cuda G, Vatrella A, Gallelli L, Caraglia M, Marra M, et al. Mitogen-activated protein kinases and asthma. J Cell Physiol. 2005. 202:642–653.




 ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download