Abstract
Gefitinib is a novel drug used to treat advanced non-small cell lung cancer. However, drug-related interstitial pneumonia is a major life-threatening side effect, which has a worldwide prevalence of 0.3-0.4%. In Japan, the prevalence is high as 3-4% but the actual frequency in Korea has not been officially assessed. We report two cases of gefitinib-induced interstitial lung disease during the treatment of non-small cell lung cancer. High-resolution computerized tomography (HRCT) of one case showed nonspecific ground glass opacity and the chest x-ray of another case showed diffuse bilateral ground glass opacity. The former patient showed a rapid good response to corticosteroid treatment whereas the latter died despite receiving aggressive treatment with high dose corticosteroid and empirical antibiotics.
Figures and Tables
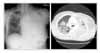 | Figure 1(A) Chest X-ray on day 25 of gefitinib therapy. Poorly defined ground glass opacities in right upper lung field has developed. (B) HRCT on day 25 of gefitinib therapy. Besides previously existed large amount of left pleural effusion and nodular thickenings, new ground glass opacity of both upper lung zone has developed. |
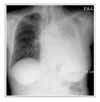 | Figure 2Chest X-ray 1 week after the discontinuation of gefitinib and steroid treatment. Ground glass opacity of right lung has improved. |
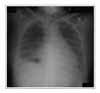 | Figure 3Chest X-ray on day 67 of gefitinib therapy. Diffuse poorly defined air-space opacities of both lung has developed. |
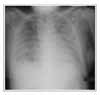 | Figure 4Chest X-ray one week after high dose steroid and antibiotics therapy. Diffuse poorly defined air-space opacities and volume reduction of both lung have markedly aggravated. |
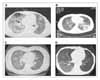 | Figure 5(A) Acute interstitial pneumonia-like observations. (B) Chronic organizing pneumonia-like observations. (C) Acute eosinophilic pneumonia-like observations. (D) Light ground-glass shadows in bilateral lung fields lacking shrinkage of lung field and traction bronchiectasis (With permission, AstraZeneca and Iressa Expert Committee, 2003). |
References
1. Arteaga CL, Johnson DH. Tyrosine kinase inhibitors-ZD1839 (Iressa). Curr Opin Oncol. 2001. 13:491–498.
2. Ciardiello F, Tortora G. A novel approach in the treatment of cancer: targeting the epidermal growth factor receptor. Clin Cancer Res. 2001. 7:2958–2970.
3. Fukuoka M, Yano S, Giaccone G, Tamura T, Nakagawa K, Douillard JY, et al. Multi-institutional randomized phase II trial of gefitinib for previously treated patients with advanced non-small-cell lung cancer. J Clin Oncol. 2003. 21:2237–2246.
4. Inoue A, Saijo Y, Maemondo M, Gomi K, Tokue Y, Kimura Y, et al. Severe acute interstitial pneumonia and gefitinib. Lancet. 2003. 361:137–139.
5. Forsythe B, Faulkner K. Safety and tolerability of gefitinib ('Iressa', ZD1839) in advanced NSCLC: overview of clinical experience. 2003b. In : Presentation at the ERS; September 27-October 1; Vienna, Austria. P327.
6. Ando M, Okamoto I, Yamamoto N, Takeda K, Tamura K, Seto T, et al. Predictive factors for interstitial lung disease, antitumor response, and survival in non-small-cell lung cancer patients treated with gefitinib. J Clin Oncol. 2006. 24:2549–2556.
7. Kang JH, Kang SY, Kim HT, Lee JS, Shim BY, Park BB, et al. Retrospective Analysis of Patients with Advanced Non-Small Cell Lung Cancer Who Participated in the Extended Access Program (EAP) of Gefitinib. In : Presentation at the CELCC; June 18-21; Praque, Czech Republic. –O20.
8. Iressa Expert Committee. Expert Committee Meeting Report. Final report on interstitial lung disease (ILD) related to gefitinib (Iressa Tablet 250). 2003. March 26; AstraZeneca.
9. Camus P, Kudoh S, Ebina M. Interstitial lung disease associated with drug therapy. Br J Cancer. 2004. 91:S18–S23.
10. Muller NL, White DA, Jiang H, Gemma A. Diagnosis and management of drug-associated interstitial lung disease. Br J Cancer. 2004. 91:S24–S30.
11. Endo M, Johkoh T, Kimura K, Yamamoto N. Imaging of gefitinib-related interstitial lung disease: multi-institutional analysis by the West Japan Thoracic Oncology Group. Lung Cancer. 2006. 52:135–140.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download