Abstract
Background
Ethyl pyruvate (EP) is a derivative of pyruvate that has recently been identified by both various in vitro and in vivo studies to have antioxidant and anti-inflammatory effects. The aim of this study was to determine the effect of EP on lipopolysaccharide (LPS)-induced acute lung injury (ALI).
Methods
5 weeks old, male BALB/c mice were used. ALI was induced by an intratracheal instillation of LPS 0.5mg/Kg/50µL of saline. The mice were divided into the control, LPS, EP+LPS, and LPS+EP groups. In the control group, balanced salt solution was injected intraperitoneally 30 minutes before or 9 hours after the intratracheal instillation of saline. In the LPS group, a balanced salt solution was also injected intraperitoneally 30 minutes before or 9 hours after instillation the LPS. In the EP+LPS group, 40mg/Kg of EP was injected 30 minutes before LPS instillation. In the LPS+EP group, 40mg/Kg of EP was injected 9 hours after LPS instillation. The TNF-α and IL-6 concentrations in the bronchoalveolar lavage fluid (BALF), and that of NF-κB in the lung tissue were measured in the control, LPS and EP+LPS groups at 6 hours after instillation of saline or LPS, and the ALI score and myeloperoxidase (MPO) activity were measured in all four groups 24 and 48 hours after LPS instillation, respectively.
Results
The TNF-α and IL-6 concentrations were significantly lower in the EP+LPS group than in the LPS group (p<0.05). The changes in the concentration of these inflammatory cytokines were strongly correlated with that of NF-κB (p<0.01). The ALI scores were significantly lower in the EP+LPS and LPS+EP groups compared with the LPS group (p<0.05). In the EP+LPS group, the MPO activity was significantly lower than the LPS group (p=0.019).
Figures and Tables
 | Figure 1Study groups and protocol. The mice were divided into control, LPS, EP+LPS, and LPS+EP groups. In the control group, balanced salt solution was injected intraperitoneally 30 minutes before or 9 hours after intratracheal instillation of saline. In the LPS group, balanced salt solution was also injected intraperitoneally 30 minutes before or 9 hours after intratracheal instillation of LPS. 40mg/Kg of EP was injected 30 minutes before LPS instillation in the EP+LPS group and was injected 9 hours after LPS instillation in the LPS+EP group. The concentration of TNF-α and IL-6 in bronchoalveolar lavage fluid (BALF), and that of NF-κB in lung tissue were measured in the control, LPS and EP+LPS groups at 6 hours after intratracheal instillation of saline or LPS, and ALI score and myeloperoxidase (MPO) activity was measured in all four groups 24 and 48 hours after LPS instillation, respectively. |
 | Figure 2The concentration of tumor necrosis factor-α (TNF-α) (A) and interleukin-6 (IL-6) (B) in bronchoalveolar lavage fluid (BALF) was significantly decreased in EP+LPS group than LPS group (*p<0.05).
ND: not detected
|
 | Figure 3The concentration of nuclear factor-κB (NF-κB) in lung homogenate of the EP+LPS group was significantly lower than the LPS group and higher than the control group (*p<0.05). It was significantly correlated with tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6) (r=Spearman's rho, **p<0.01). |
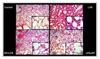 | Figure 4Histopathologic examination shows high levels of inflammatory cellular infiltration, hemorrhage, and alveolar wall thickening in the LPS group. In the EP+LPS and LPS+EP group, the degree of acute lung injury was lower and only mild inflammatory cellular infiltration was observed compared with the control group. |
 | Figure 5Acute lung injury (ALI) scores were significantly different among four groups (p=0.000 by Kruskal-Wallis test). In the EP+LPS group, the score was significantly lower than the LPS group and higher than the control group (*p<0.05). And the score of the LPS+EP group was also significantly lower compared with the LPS group (**p=0.017) without significance between the control and the EP+LPS group (p>0.05). |
References
1. Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, et al. The American-European Consensus Conference on ARDS: definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994. 149:818–824.
2. Sibille Y, Reynolds HY. Macrophages and polymorphonuclear neutrophils in lung defense and injury. Am Rev Respir Dis. 1990. 141:471–501.
3. Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000. 342:1334–1349.
4. Pugin J, Verghese G, Widmer MC, Matthay MA. The alveolar space is the site of intense inflammatory and profibrotic reactions in the early phase of acute respiratory distress syndrome. Crit Care Med. 1999. 27:304–312.
5. Janero DR, Hreniuk D, Sharif HM. Hydroperoxide-induced oxidative stress impairs heart muscle cell carbohydrate metabolism. Am J Physiol. 1994. 266:C179–C188.
6. Mohr S, Stamler JS, Brune B. Posttranslational modification of glyceraldehyde-3-phosphate dehydrogenase by S-nitrosylation and subsequent NADH attachment. J Biol Chem. 1996. 271:4209–4214.
7. Mahadev K, Zilbering A, Zhu L, Goldstein BJ. Insulin-stimulated hydrogen peroxide reversibly inhibits protein-tyrosine phosphatase 1b in vivo and enhances the early insulin action cascade. J Biol Chem. 2001. 276:21938–21942.
8. Dobsak P, Courderot-Masuyer C, Zeller M, Vergely C, Laubriet A, Assem M, et al. Antioxidative properties of pyruvate and protection of the ischemic rat heart during cardioplegia. J Cardiovasc Pharmacol. 1999. 34:651–659.
9. Salahudeen AK, Clark EC, Nath KA. Hydrogen peroxide-induced renal injury: a protective role for pyruvate in vitro and in vivo. J Clin Invest. 1991. 88:1886–1893.
10. Bunger R, Mallet RT, Hartman DA. Pyruvate-enhanced phosphorylation potential and inotropism in normoxic and postischemic isolated working heart: near-complete prevention of reperfusion contractile failure. Eur J Biochem. 1989. 180:221–233.
11. Cicalese L, Lee K, Schraut W, Watkins S, Borle A, Stanko R. Pyruvate prevents ischemia-reperfusion mucosal injury of rat small intestine. Am J Surg. 1996. 171:97–100.
12. Sileri P, Schena S, Morini S, Rastellini C, Pham S, Benedetti E, et al. Pyruvate inhibits hepatic ischemia-reperfusion injury in rats. Transplantation. 2001. 72:27–30.
13. Vonkorff RW. Pyruvate-C14, purity and stability. Anal Biochem. 1964. 8:171–178.
14. Sims CA, Wattanasirichaigoon S, Menconi MJ, Ajami AM, Fink MP. Ringer's ethyl pyruvate solution ameliorates ischemia/reperfusion-induced intestinal mucosal injury in rats. Crit Care Med. 2001. 29:1513–1518.
15. Tawadrous ZS, Delude RL, Fink MP. Resuscitation from hemorrhagic shock with Ringer's ethyl pyruvate solution improves survival and ameliorates intestinal mucosal hyperpermeability in rats. Shock. 2002. 17:473–477.
16. Ulloa L, Ochani M, Yang H, Tanovic M, Halperin D, Yang R, et al. Ethyl pyruvate prevents lethality in mice with established lethal sepsis and systemic inflammation. Proc Natl Acad Sci U S A. 2002. 99:12351–12356.
17. Yang R, Gallo DJ, Baust JJ, Uchiyama T, Watkins SK, Delude RL, et al. Ethyl pyruvate modulates inflammatory gene expression in mice subjected to hemorrhagic shock. Am J Physiol Gastrointest Liver Physiol. 2002. 283:G212–G221.
18. Yang R, Uchiyama T, Alber SM, Han X, Watkins SK, Delude RL, et al. Ethyl pyruvate ameliorates distant organ injury in a murine model of acute necrotizing pancreatitis. Crit Care Med. 2004. 32:1453–1459.
19. Pelosi P, D'Onofrio D, Chiumello D, Paolo S, Chiara G, Capelozzi VL, et al. Pulmonary and extrapulmonary acute respiratory distress syndrome are different. Eur Respir J Suppl. 2003. 42:48s–56s.
20. Rocco PR, Zin WA. Pulmonary and extrapulmonary acute respiratory distress syndrome: are they different? Curr Opin Crit Care. 2005. 11:10–17.
21. Imanaka H, Shimaoka M, Matsuura N, Nishimura M, Ohta N, Kiyono H. Ventilator-induced lung injury is associated with neutrophil infiltration, macrophage activation, and TGF-beta 1 mRNA upregulation in rat lungs. Anesth Analg. 2001. 92:428–436.
22. Hirano S. Migratory responses of PMN after intraperitoneal and intratracheal administration of lipopolysaccharide. Am J Physiol. 1996. 270:L836–L845.
23. Melzer E, Schmidt HL. Carbon isotope effects on the decarboxylation of carboxylic acids: comparison of the lactate oxidase reaction and the degradation of pyruvate by H2O2. Biochem J. 1988. 252:913–915.
24. Pelosi P, Caironi P, Gattinoni L. Pulmonary and extrapulmonary forms of acute respiratory distress syndrome. Semin Respir Crit Care Med. 2001. 22:259–268.
25. Terashima T, Matsubara H, Nakamura M, Sakamaki F, Waki Y, Soejima K, et al. Local pseudomonas instillation induces contralateral lung injury and plasma cytokines. Am J Respir Crit Care Med. 1996. 153:1600–1605.
26. Menezes SL, Bozza PT, Neto HC, Laranjeira AP, Negri EM, Capelozzi VL, et al. Pulmonary and extrapulmonary acute lung injury: inflammatory and ultrastructural analyses. J Appl Physiol. 2005. 98:1777–1783.
27. Verhasselt V, Vanden Berghe W, Vanderheyde N, Willems F, Haegeman G, Goldman M. N-acetyl-L-cysteine inhibits primary human T cell responses at the dendritic cell level: association with NF-kappaB inhibition. J Immunol. 1999. 162:2569–2574.
28. Schoonbroodt S, Ferreira V, Best-Belpomme M, Boelaert JR, Legrand-Poels S, Korner M, et al. Crucial role of the amino-terminal tyrosine residue 42 and the carboxyl-terminal PEST domain of I kappa B alpha in NF-kappa B activation by an oxidative stress. J Immunol. 2000. 164:4292–4300.
29. Livolsi A, Busuttil V, Imbert V, Abraham RT, Peyron JF. Tyrosine phosphorylation-dependent activation of NF-kappa B: requirement for p56 LCK and ZAP-70 protein tyrosine kinases. Eur J Biochem. 2001. 268:1508–1515.
30. Rahman I, Mulier B, Gilmour PS, Watchorn T, Donaldson K, Jeffery PK, et al. Oxidant-mediated lung epithelial cell tolerance: the role of intracellular glutathione and nuclear factor-kappaB. Biochem Pharmacol. 2001. 62:787–794.
31. Han Y, Englert JA, Yang R, Delude RL, Fink MP. Ethyl pyruvate inhibits nuclear factor-kappaB-dependent signaling by directly targeting p65. J Pharmacol Exp Ther. 2005. 312:1097–1105.
32. Riedemann NC, Guo RF, Ward PA. Novel strategies for the treatment of sepsis. Nat Med. 2003. 9:517–524.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download