Abstract
Background
Gefitinib (ZD 1839, Iressa) is a new anticancer agent; more specifically, it is a selective epidermal growth factor receptor tyrosine kinase inhibitor that is, widely used for various solid cancers, including lung cancer. Cutaneous adverse reactions induced by gefitinib have recently been reported; however, not much on this topic has been reported in the Korean literature.
Method
We studied cutaneous adverse reactions of gefitinib in 23 patients who suffered with non-small cell lung cancer at Chonnam National University Hwasun Hospital from October 2004 to September 2005.
Result
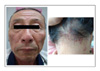
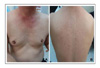
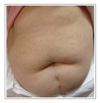
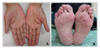
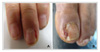
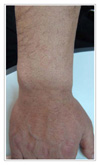
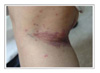
The patients ranged from 23-72 years old, and there were 17 patients with adenocarcinoma, 5 with squamous cell carcinoma and 1 with bronchioloalveolar carcinoma. The most common adverse reaction was acneiform eruptions in 15 patients (65.2%). This reaction appeared within 2 months after medication, and it didn't correlate with the therapeutic response and tumor type. Pruritus was the second most common reaction (39.1%), which was mild and generalized, especially around eyelid area. Xerosis (26.1%), exfoliation on palm and sole (21.7%), and paronychia (21.7%) followed. Hair breakage and intertrigo were rare adverse reactions.
Figures and Tables
References
1. Ranson M, Wardell S. Geftinib, a novel, orally administered agent for the treatment of cancer. J Clin Pharm Ther. 2004. 29:95–103.
2. Forsythe B, Faulkner K. Overview of the tolerability of geftinib (IRESSA) monotherapy: clinical experience in non-small-cell lung cancer. Drug Saf. 2004. 27:1081–1092.
3. Kim YJ, Hwang KC, Yu DS, Oh CH, Song HJ. A case of acneiform skin eruption associated with ZD1839 (Iressa). Korean J Dermatol. 2004. 42:478–481.
4. Bae EY, Kim MY, Kim HO, Park YM. A case of paronychia, acneiform eruption and dry skin induced bu Iressa. Korean J Dermatol. 2004. 42:665–668.
5. Yu DK, Suh DH, Youn JI. A case of hair change and acneiform eruption induced by ZD1839 (Iressa). Korean J Dermatol. 2004. 42:1461–1465.
6. Jang YH, Choi JH, Lim YH, Lee ES. Study of clinical features of cutaneous side effects associated with ZD 1839. Korean J Dermatol. 2005. 43:22–28.
7. Park JH, Oh JJ, Choi YL, Kim WS, Lee JH, Lee ES. Cutaneous adverse effects associated with Iressa (Geftinib) medication. Korean J Dermatol. 2005. 43:92–95.
8. Arteaga CL, Johnson DH. Tyrosine kinase inhibitors-ZD1839 (Iressa). Curr Opin Oncol. 2001. 13:491–498.
9. Kris M, Natale RB, Herbst R, Lynch TJ Jr, Prager D, Belani CP, et al. Efficacy of geftinib, an inhibitor of the epidermal growth factor receptor tyrosine kinase, in symptomatic patients with non-small cell lung cancer. JAMA. 2003. 290:2149–2158.
10. Albanell J, Rojo F, Averbuch S, Feyereislova A, Mascaro JM, Herbst R, et al. Pharmacodynamic studies of the epidermal growth factor receptor inhibitor ZD 1839 in skin from cancer patients: histopathologic and molecular consquencies of receptor inhibition. J Clin Oncol. 2002. 20:110–124.
11. Lee MW, Seo CW, Kim SW, Yang HJ, Lee HW, Choi JH, et al. Cutaneous side effects in non-small cell lung cancer patients treated with Iressa (ZD 1839), an inhibitor of epidermal growth factor. Acta Derm Venereol. 2004. 84:23–26.
12. Kimyai-Asadi A, Jih MH. Follicular toxic effects of chimeric anti-epidermal growth factor receptor antibody cetuximab used to treat human solid tumors. Arch Dermatol. 2002. 138:129–131.
13. Segaert S, van Cutsem E. Clinical signs, pathophysiology and management of skin toxicity during therapy with epidermal growth factor receptor inhibitors. Ann Oncol. 2005. 16:1425–1433.
14. Jacot W, Bessis D, Jorda E, Ychou M, Fabbro M, Pujol JL, et al. Acneiform eruption induced by epidermal growth factor receptor inhibitors in patients with solid tumors. Br J Dermatol. 2004. 151:238–241.
15. van Doorn R, Kirtschig G, Scheffer E, Stoof TJ, Giaccone G. Follicular and epiermal alterations in patients treated with ZD1839 (Iressa), an inhibitor of the epidermal growth factor receptor. Br J Dermatol. 2002. 147:598–601.
16. Cohen EE, Rosen F, Stadler WM, Recant W, Stenson K, Huo D, et al. Phase II study trial of ZD1839 in recurrent or metastatic squamous cell carcinoma of the head and neck. J Clin Oncol. 2003. 21:1980–1987.
17. Mohamed MK, Ramalingam S, Lin Y, Gooding W, Belani CP. Skin rash and good performance status predict improved survival with geftinib in patients with advanced non-small cell lung cancer. Ann Oncol. 2005. 16:780–785.
18. Peus D, Hamacher L, Pittelkow MR. EGF-receptor tyrosine kinase inhibition induces keratinocyte growth arrest and terminal differentiation. J Invest Dermatol. 1997. 109:751–756.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download