Abstract
Background
The overall response (20-30%) to chemotherapy in non-small cell lung cancer (NSCLC) is quite poor. Heme oxygenase-1 (HO-1) is the rate-limiting enzyme in heme degradation. There is increasing evidence suggesting that the induction of HO-1 might have an important protective effect against oxidative stress including cisplatin containing chemotherapy. This study retrospectively investigated the relationship between HO-1 expression and the response to chemotherapy containing cisplatinin advanced NSCLC patients.
Material and Methods
The medical records including the responses to chemotherapy of fifty nine cases were evaluated retrospectively, and the tissue samples of these patients were immunohistochemically stained for HO-1.
Figures and Tables
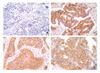 | Figure 1Immunohistochemical staining for HO-1. (A) negative HO-1 expression (×200), (B) positive HO-1 expression in adenocarcinoma (×200), (C) positive HO-1 expression in squamous cell carcinoma (×200), (D) positive HO-1 expression in non-small cell lung cancer (×200). |
References
1. Korea National Statistical Office. Deaths and death rate by cause. 2004.
2. Minna JD. Neoplasms of the lung. Harrison's principles of internal medicine. 2005. 16th ed. New York: McGraw-Hill;506–515.
3. Scientific Committee of Korean Academy of Tuberculosis and Respiratory Diseases. The national survey of lung cancer in Korea. Tuberc Respir Dis. 1999. 46:455–465.
4. Graziano SL. Non-small cell lung cancer: clinical value of new biological predictors. Lung cancer. 1997. 17:S37–S58.
5. Fang J, Akaike T, Maeda H. Antiapoptotic role of heme oxygenase(HO) and the potential of HO as a target in anticancer treatment. Apoptosis. 2004. 9:27–35.
6. Liu ZM, Chen GG, Ng EK, Leung WK, Sung JJ, Chung SS. Upregulation of heme oxygenase-1 and p21 confers resistance to apoptosis in human gastric cancer cells. Oncogene. 2004. 23:503–513.
7. Agarwal A, Balla J, Alam J, Croatt AJ, Nath KA. Induction of heme oxygenase in toxic renal injury: a protective role in cisplatin nephrotoxicity in the rat. Kidney Int. 1995. 48:1298–1307.
8. Fang J, Sawa T, Akaike T, Greish K, Maeda H. Enhancement of chemotherapeutic response of tumor cells by a heme oxygenase inhibitor, pegylated zinc protoporphyrin. Int J Cancer. 2004. 109:1–8.
9. Mountain CF. Revisions in the international system for staging lung cancer. Chest. 1997. 111:1710–1717.
10. Miller AB, Hoogstraten B, Staquet M, Winkler A. Reporting results of cancer treatment. Cancer. 1981. 47:207–214.
11. Tenhunen R, Marver HS, Schmid R. The enzymatic conversion of heme to bilirubin by microsomal heme oxygenase. Proc Natl Acad Sci U S A. 1968. 61:748–755.
12. Alam J. Heme oxygenase-1: past, present, and future. Antioxid Redox Signal. 2002. 4:559–562.
13. Stocker R, MacDonagh AF, Glazer AN, Ames BN. Antioxidant activities of bile pigments: biliverdin and bilirubin. Methods Enzymol. 1990. 186:301–309.
14. Verma A, Hirsch DJ, Glatt CE, Ronnett GV, Snyder SH. Carbon monoxide: a putative neural messenger. Science. 1993. 259:381–384.
15. Applegate LA, Luscher P, Tyrrell RM. Induction of heme oxygenase: a general response to oxidant stress in cultured mammalian cells. Cancer Res. 1991. 51:974–978.
16. Nath KA, Balla G, Vercellotii GM, Balla J, Jacob HS, Levitt MD, et al. Induction of heme oxygenase is a rapid, protective response in rhabdomyolysis in the rat. J Clin Invest. 1992. 90:267–270.
17. Tanaka S, Akaike T, Fang J, Beppu T, Ogawa M, Tamura F, et al. Antiapoptotic effect of haem oxygenase-1 induced by nitric oxide in experimental solid tumour. Br J Cancer. 2003. 88:902–909.
18. Doi K, Akaike T, Fujii S, Tanaka S, Ikebe N, Beppu T, et al. Induction of heam oxygenase-1 by nitric oxide and ischaemia in experimental solid tumors and implications for tumour growth. Br J Cancer. 1999. 80:1945–1954.
19. Agarwal A, Balla J, Alam J, Croatt AJ, Nath KA. Induction of heme oxygenase in toxic renal injury: a protective role in cisplatin nephrotoxicity in the rat. Kidney Int. 1995. 48:1298–1307.
20. Yokoyama S, Mita S, Okabe A, Abe M, Ogawa M. Prediction of radiosensitivity in human esophageal squamous cell carcinomas with heme oxygenase-1: a clinicopathologic and immunohistochemical study. Oncol Rep. 2001. 8:355–358.
21. Yanagawa T, Omura K, Harada H, Nakaso K, Iwasa S, Koyama Y, et al. Heme oxygenase-1 expression predicts cervical lymph node metastasis of tongue squamous cell carcinoma. Oral Oncol. 2004. 40:21–27.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download