Abstract
Pulmonary arterial hypertension (PAH) is often difficult to diagnose and challenging to treat. Untreated, it is characterized by a progressive increase in pulmonary vascular resistance leading to right ventricular failure and death. The past decade has seen remarkable improvements in therapy, driven largely by the conduct of randomized controlled trials. Still, the selection of most appropriate therapy is complex, and requires familiarity with the disease process, evidence from treatment trials, complicated drug delivery systems, dosing regimens, side effects, and complications. We tried to provide evidence‐based treatment recommendations for physicians involved in the care of these complex patients. Due to the complexity of the diagnostic evaluation required, and the treatment options available, it is strongly recommended that consideration be given to referral of patients with PAH to a specialized center.
REFERENCES
1.Weir EK., Rubin LJ., Ayres SM., Bergofsky EH., Brundage BH., Detre KM, et al. The acute administration of vasodilator s in primary pulmonary hypertension: experience from the National Institutes of Health Registry on Primary Pulmonary Hypertension. Am Rev Respir Dis. 1989. 140:1623–30.
2.S. Kaufmann E., Levy PS. The effect of high doses of calcium-channel blockers on survival in primary pulmonary hypertension. N Engl J Med. 1992. 327:76–81.

3.Christman BW., McPherson CD., Newman JH., King GA., Bernard GR., Groves BM, et al. An imbalance between the excretion of thromboxane and prostacyclin metabolites in pulmonary hypertension. N Engl J Med. 1992. 327:70–5.

4.Tuder RM., Cool CD., Geraci MW., Wang J., Abman SH., Wright L, et al. Prostacyclin synthase expression is decreased in lungs from patients with severe pulmonary hypertension. Am J Respir Crit Care Med. 1999. 159:1925–32.

5.RJ. Rubin LJ., Long WA., McGoon MD., Rich S., Ba-desch DB, et al. A comparison of continuous intravenous epoprostenol (prostacyclin) with conventional therapy for primary pulmonary hypertension. N Engl J Med. 1996. 334:296–302.

6.DB. Tapson VF., McGoon MD., Brundage BH., Rubin LJ., Wigley FM, et al. Continuous intravenous epoprostenol for pulmonary hypertension due to the scleroderma spectum of disease: a randomized, controlled trial. Ann Intern Med. 2000. 132:425–34.
7.G. Barst RJ., Galie N., Naeije R., Rich S., Bourge RC, et al. Continuous subcutaneous infusion of trepros-timil, a prostacyclin analogue, in patients with pulmonary arterial hypertension: a doubleblind, randomized, placebo-controlled trial. Am J Respir Crit Care Med. 2002. 165:800–4.
8.Krause W., Krais T. Pharmacokinetics and pharmacodynamics of the prostacyclin analogue iloprost in man. Eur J Clin Pharmacol. 1986. 30:61–8.

9.MM. Olschewski H., Ghofrani HA., Wilkens H., Winkler J., Borst MM, et al. A comparison of the acute hemodynamic effects of inhaled nitric oxide and aerosolized iloprost in primary pulmonary hypertension. J Am Coll Cardiol. 2000. 35:176–82.
10.T. Schmehl T., Hoeper MM., Rose F., Ghofrani HA., Olschewski H, et al. Ultrasonic versus jet nebulization of iloprost in severe pulmonary hypertension. Eur Respir J. 2001. 17:14–9.
11.H. Simonneau G., Galie N., Higenbottam T., Naeije R., Rubin LJ, et al. Inhaled iloprost for severe pulmonary hypertension. N Engl J Med. 2002. 347:322–9.

12.Y. Yoshioka T., Shimouchi A., Satoh T., Kunieda T. Orally active prostacyclin analogue in primary pulmonary hypertension. Lancet. 1997. 349:1365.
13.N. Uematsu M., Okano Y., Satoh T., Kyotani S., Sakamaki S, et al. Effect of orally active prostacyclin analogue on survival of outpatients with primary pulmonary hypertension. J Am Coll Cardiol. 1999. 34:1–188. -92.
14.Galie N., Humber M., Vachiery JL., Vizza CD., Kneussl M., Manes A, et al. Effects of beraprost sodium, an oral prostacyclin analogue, in patients with pulmonary arterial hypertension: a randomized, doubleblind, placebo-controlled trial. J Am Coll Cardiol. 2002. 39:1496–502.

15.RJ. McGoon M., McLaughlin V., Tapson V., Rich S., Rubin L, et al. Beraprost therapy for pulmonary arterial hypertension. J Am Coll Cardiol. 2003. 41:2119–25.
16.MR. Endothelin-1: a mediator of pulmonary hypertension? Pulm Pharmacol Ther. 1998. 11:125–32.
17.A. Remuzzi G. Endothelin antagonists. Lancet. 1999. 353:133–8.
18.SJ. Chen YF., Meng QC., Durand J., Dicarlo VS., Oparil S. Endothelin-receptor antagonist bosentan prevents and reverses hypoxic pulmonary hypertension in rats. J Appl Physiol. 1995. 79:2122–31.

19.NS. Warburton RR., Pietras L., Klinge JR. Nonspecific endothelin-receptor antagonist blunts monocrotaline-in-duced pulmonary hypertension in rats. J Appl Physiol. 1997. 83:1209–15.
20.JM. Wellmann SA., McNamara JL., Lombardi JP., Wagner CJ., Raake JL, et al. Bosentan prevents hypoxia-reoxygenation-induced pulmonary hypertension and improves pulmonary function. Ann Thorac Surg. 1999. 68:1714–21.
21.C. Chan MF., Stavros F., Raju B., Okun I., Mong S, et al. Discovery of TBC11251, a potent, long acting, orally active endothelin receptor-A selective antagonist. J Med Chem. 1997. 40:1690–7.
22.RN. Simonneau G., Sitbon O., Robbins IM., Frost A., Tapson VF, et al. Effects of the dual endothelin-receptor antagonist bosentan in patients with pulmonary hypertension: a randomised placebo-controlled study. Lancet. 2001. 358:1119–23.
23.Barst RJ., Langleben D., Frost A., Horn EM., Oudiz R., Shapiro S, et al. Sitaxsentan therapy for pulmonary arterial hypertension. Am J Respir Crit Care Med. 2004. 169:441–7.

24.Hanson KA., Burns F., Rybalkin SD., Miller JW., Beavo J., Clarke WR. Developmental changes in lung cGMP phosphodiesterase-5 activity, protein, and message. Am J Respir Crit Care Med. 1998. 158:279–88.

25.Hanson KA., Ziegler JW., Rybalkin SD., Miller JW., Abman SH., Clarke WR. Chronic pulmonary hypertension increases fetal lung cGMP phosphodiesterase activity. Am J Physiol. 1998. 275:L931–41.
26.Braner DA., Fineman JR., Chang R., Soifer SJ. M&B 22948, a cGMP phosphodiesterase inhibitor, is a pulmonary vasodilator in lambs. Am J Physiol. 1993. 264:H252–8.
27.McMahon TJ., Ignarro LJ., Kadowitz PJ. Influence of Zaprinast on vascular tone and vasodilator responses in the cat pulmonary vascular bed. J Appl Physiol. 1993. 74:1704–11.

28.Clarke WR., Uezono S., Chambers A., Doepfner P. The type III phosphodiesterase inhibitor milrinone and type V PDE inhibitor dipyridamole individually and synergistically reduce elevated pulmonary vascular resistance. Pulm Pharmacol. 1994. 7:81–9.

29.Cohen AH., Hanson K., Morris K., Fouty B., McMurty IF., Clarke W, et al. Inhibition of cyclic3'-5'-guanosine monophosphate-specific phosphodiesterase selectively vasodilates the pulmonary circulation in chronically hypoxic rats. J Clin Invest. 1996. 97:172–9.
30.Thusu KG., Morin FC 3rd., Russell JA., Steinhorn RH. The cGMP phosphodiesterase inhibitor zaprinast enhances the effect ofnitric oxide. Am J Respir Crit Care Med. 1995. 152:1605–10.
31.Ziegler JW., Ivy DD., Fox JJ., Kinsella JP., Clarke WR., Abman SH. Dipyridamole potentiates pulmonary vasodilation induced by acetylcholine and nitric oxide in the ovine fetus. Am J Respir Crit Care Med. 1998. 157:1104–10.

32.Ghofrani HA., Wiedemann R., Rose F., Olschewski H., Schermuly RT., Weissmann N, et al. Combination therapy with oral sildenafil and inhaled iloprost for severe pulmonary hypertension. Ann Intern Med. 2002. 136:515–22.

33.E. Tymchak W., Lien D., webster L., Hashimoto K., Archer S. Oral sildenafil is an effective and specific pulmonary vasodilator in patients with pulmonary arterial hypertension: comparison with inhaled nitric oxide. Circulation. 2002. 105:2398–403.
34.JJ. Maroo A., Pereira NL., Ginns LC., Dec GW., Zapol WM, et al. Effect of sildenafil on the acute pulmonary vasodilator response to inhaled nitric oxide in adults with primary pulmonary hypertension. Am J Cardiol. 2002. 90:677–80.
35.AM. Wessel DL. Sildenafil ameliorates effects of inhaled nitric oxide withdrawal. Anesthesiology. 1999. 91:307–10.
36.Wilkens H., Guth A., Konig J., Forestier N., Cremers B., Hennen B, et al. Effect of inhaled iloprost plus oral sildenafil in patients with primary pulmonary hypertension. Circulation. 2001. 104:1218–22.

37.SH. Charfield BA., Hall SL., McMurtry IF. Role of endothelium-derived relaxing factor during transition of pulmonary circulation at birth. Am J Physiol. 1990. 259:H1921–7.
38.Villamor E., Le Cras TD., Horan MP., Halbower AC., Tuder RM., Abman SH. Chronic intrauterine pulmonary hypertension impairs endothelial nitric oxide synthase in the ovine fetus. Am J Physiol. 1997. 27–2. L1013-20.

39.KA. Fouty BW., Tyler RC., Morris KG Jr., Hepler LK., Sato K, et al. The pulmonary circulation of homozygous or heterozygous eNOS-null mice is hyperresponsive to mild hypoxia. J Clin Invest. 1999. 103:291–9.
40.JP. Dzau VJ. Nitric oxide synthase: role in the genesis of vascular disease. Annu Rev Med. 1997. 48:4–89. -509.
41.RD,Shesely EG., Maeda N., Smithies O., Segal SS., Sessa WC. Direct evidence for the importance of endothelium-derived nitric oxide in vascular remodeling. J Clin Invest. 1998. 101:731–6.
42.Loscalzo J., Welch G. Nitric oxide and its role in the cardiovascular system. Prog Cardiovasc Dis. 1995. 38.

43.Buechler WA., Ivanova K., Wolfram G., Drummer C., Heim TM., Gerzer R. Soluble guanylyl cyclase and platelet function. Am N Y Acad Sci. 1994. 714:151–7.
44.Radomski MW., Moncada S. The biological and pharmacological role of nitric oxide in platelet function. Adv Exp Med Biol. 1993. 344:251–64.

45.Zapol WM., Falke KJ., Hurford WE., Roberts JD Jr. Inhaling nitric oxide: a selective pulmonary vasodilator and bronchodilator. Chest. 1994. 105:87S–91S.

46.JP. Neish SR., Shaffer E., Abman SH. Low-dose inhalation nitric oxide in persistent pulmonary hypertension of the newborn. Lancet. 1992. 340:819–20.
47.The Neonatal Inhaled Nitric Oxide Study Group. Inhaled nitric oxide in full-term and nearly full-term infants with hypoxic respiratory failure. N Engl J Med. 1997. 336:597–604.
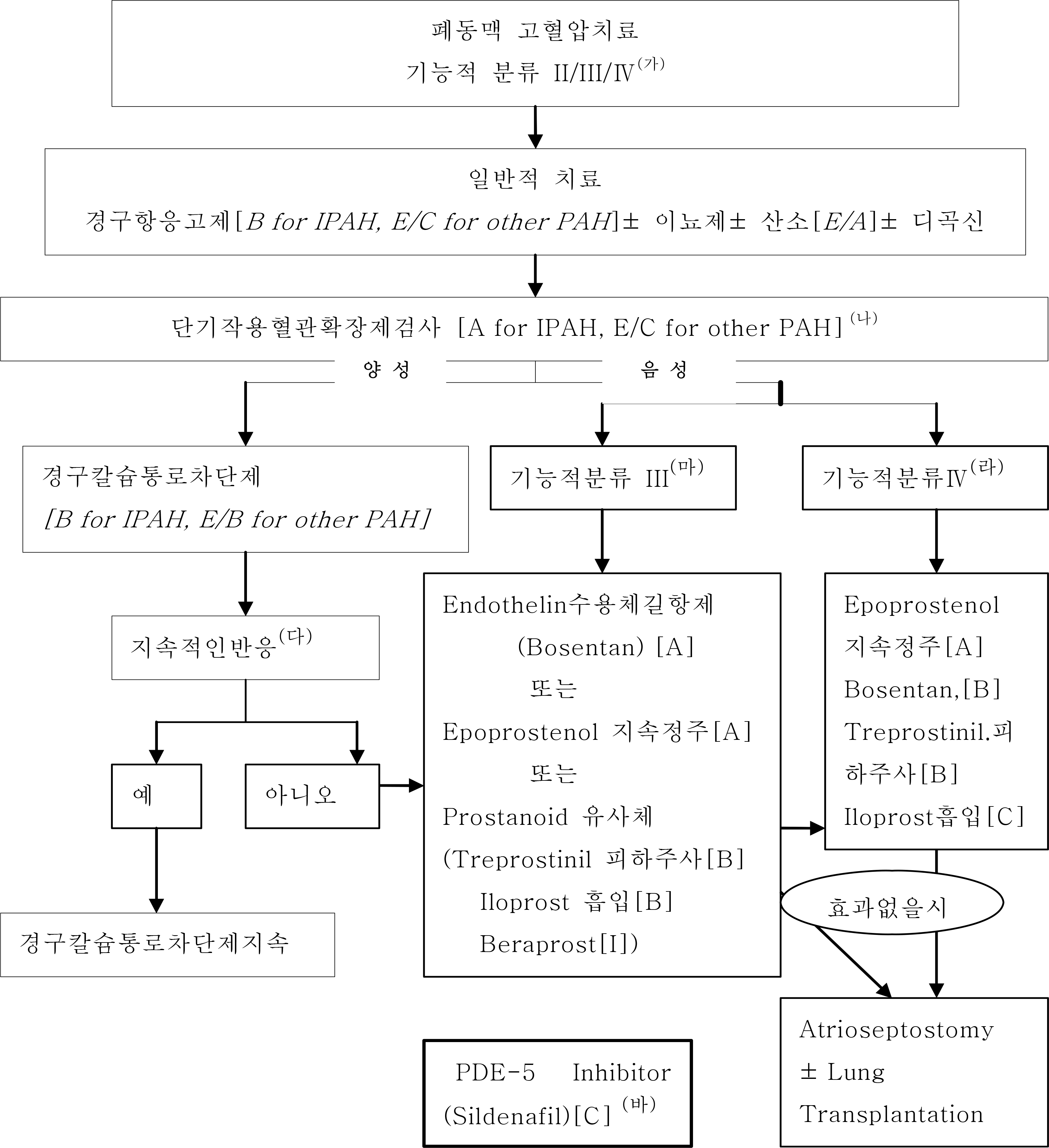
Figure 1.
특발성 폐동맥고혈압 환자의 치료:“제3회 폐동맥고혈압 세계심포지엄 2003-07-23, 베니스”(가) 치료 알고리듬은 뉴욕심장협회 기능적분류 III, Ⅳ기 환자에 중점을 두고 있다. II기 환자는 칼슘통로 차단제 치료에 적합하지 않거나 실패한자로 치료에 효과가 있을 것으로 보고 있다. (나) 단기작용 혈관확장제 검사에서 유의한 반응은 산화질소 흡입또는, epoprostenol 또는 Adenosine 정맥주사시 평균 폐동맥압이 10mmHg 이상 감소하여 40mmHg 이하를 유지하는것으로 정의한다. 이때 심박출량은 변화하지 않거나 증가하여야 한다. (다) 경구칼슘통로차단제에 대한 지속적인 반응은 치료수개월 후 뉴욕심장협회 기능적 분류가 I 또는 Ⅱ 로 향상되며 혈역학적으로 거의 정상화 되는 것으로 정의한다. (라) 뉴욕심장협회 기능적 분류 Ⅳ의 불안정한 환자는 Epoprostenol정주를 최우선의 치료로 권장한다. (마) 뉴욕심장협회 기능적 분류 III 환자의 첫번째 치료에는 엔도텔린수용체 길항제 (Bosentan) 또는 Epoprostenol 지속정주 또는 프로스타노이드 유사체 (Treprostinil 피하주사, Iloprost 흡입)를 포함시킨다. (바) 모든 치료약제에 실패하거나, 적합하지 않은 폐동맥 고혈압환자 는 sildenafil 을 고려한다. Grade of Recommendation :Noted in []: A, strong; B, moderate; C, weak; D,negative; I, inconclusive (no recommendation possible); E/A, strong recommendation based on expert opinion only; E/B, moderate recommendation based on expert opinion only; E/C, weak recommendation based on expert opinion only; E/D, negative recommendation based on expert opinion only. IPAH= Idiopathic pulmonary arterial hypertension; PAH= Pulmonar arterial hypertension; PDE= Phosphodiesterase.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download