Abstract
Peroxiredoxin I (Prx I) is a member of the peroxiredoxins (Prxs) family, which are antioxidant enzymes that regulate various cellular process via intracellular oxidative signal pathways. In order to investigate the correlation between Prx I and the γ-secretase complex, which causes Alzheimer's disease (AD), the expression level of Prx I was firstly evaluated in an animal model for AD. NSE/hPen-2 transgenic (Tg) mice, which were used as animal model in this study, showed a high level of Pen-2 expression and accumulation of Aβ-42 peptides in the hippocampus of brain. The expression level of Prx I was significantly higher on the mRNA and protein level in the brain of this model, while not change in Prx VI expression was observed. Furthermore, to verify the effect of Prx I on the γ-secretase components in vitro, the expression level of these components was analyzed in the Prx I transfectants. Of the components of the γ-secretase complex, the expression of PS-2 and Pen-2 was lower in the transfectants overexpressing Prx I compared to the vector transfectants. However, the expression of APP, NCT and APH-1 did not change in Prx I transfectants. Therefore, these results suggested that the expression of Prx I may be induced by the accumulation of Aβ-42 peptides and the overexpression of Prx I in neuroblastoma cells may regulate the expression of γ-secretase components.
Peroxiredoxins (Prxs) are a 24-kDa peroxidase that belongs to an antioxidant enzyme family. The cysteine (Cys) residue on this protein is the primary site of oxidation and acts as an electron donor for the reduction of peroxides [1,2]. Six isoforms of mammalian Prxs (I-VI) were identified with similar immunological properties and amino acid sequences [3,4]. The distribution of the Prxs isoforms in the human brain was found to vary. Prx I was primarily expressed in astrocytes, while Prx II was expressed in neurons of various region including the cerebral cortex, hippocampus, cerebellum, basal ganglia, substantia and spinal cord. Moreover, they were differentially located within cells. Prx I, II and VI were mainly distributed to the cytosol, but Prx III and V were largely present in the organelles and Prx IV was secreted into the extracellular region [5,6].
Of the six isoforms, Prx I was predominantly expressed in various type of tumors and functioned as an anti-apoptotic protein for tumor cells proliferation and survival [2]. In addition, several studies have found that Prx I was tightly correlated with neurodegenerative disease [7,8]. The expression level of Prx I was not significantly altered in Down syndrome (DS), Alzheimer's disease (AD) and Pick's disease (PD) when compared to the control [7]. Especially, AD which was showed the massive accumulation of extracellular Aβ-42 peptides produced by γ-secretase composing of four subunits and the hyperphosphorylation of Tau proteins has been received great interest from scientists [9,10]. The Aβ-resistance PC12 cell line showed higher expression levels of multiple Prxs isoforms than that of the control cells with reduced cysteine oxidation. Furthermore, an increase in Aβ-resistant was induced by transfection of wild type Prx I in PC12 cells and rat primary hippocampal neurons [8]. However, the effects of Prx I on the expression of the γ-secretase complex on AD have not yet been studied and are unknown. Therefore, in this study, we investigated whether Prx I could regulate the expression of the γ-secretase complex, which causes AD, in cells overexpressing Prx I and an AD animal model.
The animal model for AD was produced by the microinjection of the human Pen-2 gene, a key regulator of γ-secretase complex, into the pronucleus of fertilized eggs as described previously [11]. All animal experimental procedures were approved by the Institutional Animal Care and Use Committee (IACUC) at the Pusan National University (Approval No.: PNU-2010-000220). All mice were supplied by the breeding center of Korea FDA facility and were housed in cages under a strict light cycle (lights on at 06:00 h and off at 18:00 h) and constant temperature of 23±1℃. In addition, all mice were provided a standard irradiated chow diet (Purina Mills, St. Louis, MO, USA) ad libitum and maintained in a specific pathogen free (SPF) state.
For the preparation of total RNA, tissues frozen in liquid nitrogen were chopped with scissors and homogenized in a RNA-Bee™ solution (Tel-Test, Austin, TX, USA). The isolated RNA was then quantified using an Ultraspec 1000 system (Amersham Pharmacia Biotech, Buckinghamshire, UK). To characterize the expression of transgenes, RT-PCR was conducted using 5 µg of total RNA from each of the tissue samples. 500 ng of Oligo-dT primer (Invitrogen, Carlsbad, CA, USA) was annealed for 10 min at 70℃. Complementary DNA, which was utilized as a template for further amplification, was synthesized via the addition of dATP, dCTP, dGTP and dTTP, as well as 200 units of reverse transcriptase. In these reactions, 10 pmoles of the sense and antisense primers were added, and the reaction mixtures were subjected to 30 cycles of amplification. Amplification was conducted in the aforementioned thermal cycler under the following conditions: 30 sec at 94℃, 30 sec at 62℃ and 45 sec at 72℃. In each case, minus-RT controls were included to distinguish between the DNA and RNA products. This experiment was repeated three times, and the relative differences in RNA quantity were also reproducibly observed in the three experiments. The sequences of the sense and antisense primers for Pen-2 were 5'-GCTAT GAACC TGGAG CGAGT G-3' and 5'-GAAGG AGAGG TAGTC CCCAA GG-3', Prx I were 5'-GCGCT AGCGG ACTGC TGATA GGAAG ATGTC-3' and 5'-GCCTC GAGCA GCGCT CACTT CTGCT TGGAG-3', Prx VI were 5'-GCGCT AGCCT TGTTC TCAGC GTCAC CACTG-3' and 5'-GCCTC GAGCC AGTAC TGGAT GTGCA GATGC AG-3', β-actin were 5'-TGGAA TCCTG TGGCA TCCAT GAAAC-3' and 5'-TAAAA CGCAG CTCAG TAACA GTCCG-3', respectively. Finally, the levels of each Pen-2, Prx I and Prx VI RT-PCR product were quantified using the aforementioned electrophoresis documentation and analysis system on a 1% agarose gel.
SH-SY5Y cells harvested from 100 mm-diameter culture dishes and the tissue from non-Tg and NSE/hPen-2 Tg mice were solubilized and homogenized with 1% nonidet P-40 in 150 mM NaCl, 10 mM Tris HCl (pH 7.5), and 1 mM EDTA, and supplemented with a protein inhibitor mixture (Roche, Basel, Switzerland). From 15 to 30 µg of protein was separated by electrophoresis on a 10% polyacrylamide gel for 2 h and the resolved species were transferred to a nitrocellulose membrane by electroblotting for 2 h. The membrane was incubated with primary anti-human Pen-2 antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA, 1:1,000 dilution), anti-PS-2 antibody (Cell Signaling Technology, Boston, MA, USA, 1:1,000 dilution), anti-APP antibody (Sigma-Aldrich, St. Louis, MO, USA, 1:4,000 dilution), anti-APH-1 antibody (Sigma-Aldrich, 1:1,000 dilution), anti-NCT antibody (Cell Signaling Technology, 1:1000 dilution), anti-Prx I antibody (Abcam, Cambridge, UK, 1:1000 dilution), anti-Prx VI antibody (Abcam, 1:1000 dilution) or anti-actin antibody (Sigma-Aldrich, 1:3,000 dilution) overnight at 4℃. Each membrane was washed with buffer (137 mM NaCl, 2.7 mM KCl, 10 mM NaHPO4, and 0.05% Tween-20) and incubated with a 1:1,000 dilution of horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG at room temperature for 2 h. The membrane blots were developed using Enhanced Chemiluminescence Reagent Plus kit (Amersham).
Brain perfusion and immunohistochemical analyses was performed as previously described [12,13]. Briefly, mice were anaesthetised with Zoletil 50 (Virbac, Carros cedex, France) and transcardially perfused with 1X PBS followed by 4% formaldehyde to effectively remove the blood and fix the brain tissue. After perfusion, each mouse brain was isolated from the skull and fixed overnight in formaldehyde. Each brain was dehydrated and embedded in paraffin. A series of brain sections (10 µm) were cut from paraffin-embedded tissue using a Leica microtome (Leica Microsystems, Bannockbrun, IL, USA). For immunohistochemical analysis, these sections were de-paraffinized with xylene, rehydrated and pretreated for 30 min at room temperature with PBS blocking buffer containing 10% goat serum. The sections were then incubated with the anti-Aβ-42 antibody (Invitrogen), at a dilution of 1:100 in PBS blocking buffer. The antigen-antibody complexes were visualized with biotinylated secondary antibody (goat anti-rabbit)-conjugated HRP streptavidin (Histostain-Plus Kit; Zymed, South San Francisco, CA, USA), at a dilution of 1:1,500 in PBS blocking buffer. The Aβ-42 peptides were detected using stable 3,3'-diaminobenzidine (DAB; Invitrogen) and observed using a model BX50F-3 optical microscope (Olympus, Tokyo, Japan).
The CMV/hPrx I plasmid, which harbors hPrx I under the control of the CMV promoter, was constructed. Briefly, the hPrx I gene (GenBank accession No. BC021683.1) was amplified by the PCR using a full-length RNA isolated from NCI-H460 human carcinomal cells. The primers used for the amplification were hPrx I sense primer, 5'-GCgctagc GGACT GCTGA TAGGA AGATG TC-3' (italic letter, NheI site; ATG, start codon; capital letter, hPrx I sense primer corresponding to nucleotides 57-78 of hPrx I) and the hPrx I anti-sense primer, 5'-GCctcgag CAGCG CTCAC TTCTG CTTGG AG-3' (italic letter, XhoI site; the capital letter corresponds to nucleotides 658-679 of hPrx I). The primers used contained a recognition sequence for the NheI and XhoI enzymes at the 5' and 3' end of the PCR products, respectively. The amplified hPrx I was 622-bp in length, and the product was cloned into the T cloning vector (pGEM-T; Promega, Madison, WI, USA). Sequence analysis was conducted in order to confirm whether or not the cloned hPrx I sequence was identical to hPrx I cDNA, and the resulting sequence was aligned with the NCBI sequence database, using the BLAST program to identify their corresponding hPrx I gene. The hPrx I cDNA fragment isolated using NheI and XhoI digestion was inserted into the pEGFP-C1 vector in order to construct the CMV/hPrx I recombinant vector. Finally, the CMV/hPrx I plasmid was subjected to enzyme digestion and automatic sequence analysis to confirm their sequence and orientation (Figure 3A).
NCI-H460 cells and SH-SY5Y cells were purchased from the Korean Cell Line Bank (Seoul, Korea). Cells were grown in monolayers in RPMI 1640 (Hyclone Co., Logan, UT, USA) supplemented with 10% fetal bovine serum (FBS; Invitrogen) and antibiotics (100 unit/mL penicillin and 100 µg/mL streptomycin; Invitrogen) during incubation at 37℃ in a humidified incubator containing 5% CO2 in air. All other chemicals were purchased from Sigma-Aldrich. To overexpress the Prx I protein in neuroblastoma cells, the cells were transfected with the CMV/hPrx I plasmid using the Lipofectamine™ Reagent (Invitrogen). SH-SY5Y cells were seeded at a density of 2×106 cells per 100 mm-diameter dish. After reaching 80-90% confluence, a plasmid-lipofectamine mixture containing 20 µg of DNA (per dish) was added, and the cells were incubated for an additional 5 h at 37℃ in OptiMEM medium. The cells were then cultured in fresh serum-free medium for 24 h and were used for Western blot and flow cytometry analyses.
Remarkable accumulation of Aβ-42 peptides is one of most important features in the animal model for AD [12]. Pen-2 expression was first detected using RT-PCR analysis. The mRNA expression of Pen-2 was dramatically higher in the brain of NSE/hPen-2 Tg mice (Figure 1A). In order to investigate the accumulation of Aβ-42 peptides in the brain of NSE/hPen-2 Tg mice, the level of Aβ-42 peptides was detected by immunohistochemical analysis. NSE/hPen-2 Tg mice contained higher levels of Aβ-42 peptides in the hippocampus of brain than the Non-Tg mice (Figure 1B). Therefore, the NSE/hPen-2 Tg mice used in this study had the distinct features of AD.
Next, to investigate whether accumulation of Aβ-42 peptides could alter Prx I and VI expression, the Prx I and VI mRNA and protein levels were detected in the brain of Non-Tg mice and NSE/hPen-2 Tg mice. The expression level of Prx I mRNA was significantly higher in NSE/hPen-2 Tg mice compared to Non-Tg mice, while the expression level of Prx VI was not different between the two groups (Figure 2A). Also, the protein and mRNA levels of both enzymes were very similar, although the ratio was different in each analysis (Figure 2A). These results suggested that accumulation of Aβ-42 peptides induced by Pen-2 overexpression may upregulate Prx I but not Prx VI.
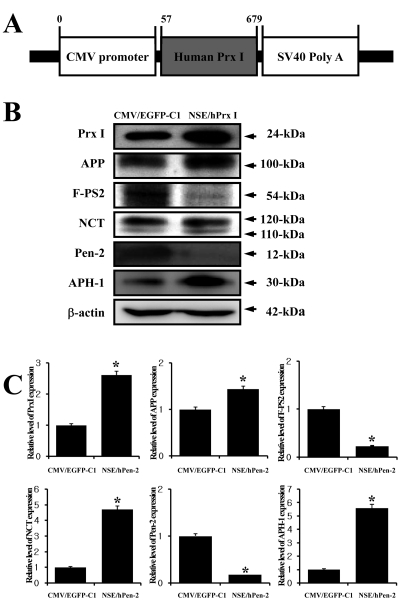
To study the effect of Prx I on the expression of the γ-secretase complex, SH-SY5Y neuroblastoma cells were transfected with the CMV/hPrx I plasmid and the expression level of each component from γ-secretase was detected using the specific antibody. The expression level of Prx I was successfully increased in the CMV/hPrx I transfectants when compared to the vector transfectants. In addition, the expression level of APP, which is a substrate of γ-secretase, was slightly higher in the CMV/hPrx I transfectants than the vector transfectants. Of the different components of the γ-secretase complex, the expression level of key factors including PS-2 and Pen-2 was lower in the CMV/hPrx I transfectants when compared to the vector transfectants, while the expression level of the assistant factor (APH-1 and NCT) was higher in the CMV/hPrx I transfectants. These results suggested that Prx I may down-regulate PS-2 and Pen-2 and up-regulate APH-1 and NCT of the γ-secretase complex.
Prx I is a member of the 2-Cys subfamily and is considered a candidate therapy target of lung adenocarcinoma since overexpression of Prx I has been observed in various cancer cell lines [14,15]. However, most of studies have focused on the role of Prx I in the development, progression and drug resistance of cancer [14,16]. Only a few studies have examined the correlation between Prx I and neurodegenerative diseases. In this study, we demonstrated that Prx I could regulate the expression of key molecules responsible for neurodegenerative diseases.
Especially, the expression level of PS-1 protein, a component of the γ-secretase complex, was strongly correlated with oxidative stress. In both the cells lysate and lipid raft fraction of neuroblastoma SH-SY5Y cells, a significant increase in PS-1 protein expression was observed in cells subjected to oxidative stress; however, under the same conditions, no changes in the expression of NCT, APH-1, Pen-2 or BACE-1 were observed. Furthermore, this increase in PS-1 protein expression was prevented by co-treatment with an antioxidant [17]. In this study, an antioxidant condition was induced by transfection of CMV/hPrx I vector, which altered the expression level of γ-secretase complex components. As shown in Fig. 3, the expression level of the NCT and APH-1 protein was significantly increase by the overexpression of Prx I. However, PS-2 and Pen-2, which is a coregulator of γ-secretase activity, was higher under the same condition. Therefore, our results showed that the expression level of the γ-secretase complex components may be altered by oxidative stress conditions as well as the overexpression of antioxidant enzymes.
Several studies had provided conflicting results on the expression levels of Prxs in the brain of AD. In a study that examined Prxs expression in three neurodegenerative diseases, Down syndrome (DS), AD and Pick's disease (PD), the expression level of Prx I under the disease condition were reported to not be significantly different from the control. However, the expression level of Prx II was found to be dramatically higher in the frontal cortex for these three diseases, where Prx III expression was lower in same region of DS and PD [7]. Similar results were observed in Tg mice overexpression Aβ-42 peptides and amyloid binding alcohol dehydrogenase. Proteomic analysis of the brain from these mice showed that the Prx II level was higher in the AD mice model [18]. However, another study reported that the expression level of Prx I was higher in post-morten human AD cortex tissue than in age-matched control [8]. In our study, the expression level of Prx I was significantly higher in Tg mice overexpressing Pen-2 gene compare to non-Tg mice, while the level of Prx VI was the same between these two groups. Therefore, our results suggested that Prx I may be correlated with the pathological condition of AD.
In this study, we investigated the correlation between Prx I and the pathogenesis of AD. These results showed that the accumulation of Aβ pepetides induced the expression of Prx I, but not Prx VI. Also, the overexpression of Prx I by transfection induced an increase in NCT and APH-1 expression and a decrease in PS-2 and Pen-2 expression.
References
1. Hoidal JR. Reactive oxygen species and cell signaling. Am J Respir Cell Mol Biol. 2001; 25(6):661–663. PMID: 11726388.

2. Luo Y, Pang H, Li S, Cao H, Peng Z, Fan C, Li S. Production and radioimmunoimaging of novel fully human phage display recombinant antibodies and growth inhibition of lung adenocarcinoma cell line overexpressing Prx I. Cancer Biol Ther. 2009; 8(14):1369–1377. PMID: 19556853.

3. Chae HZ, Kang SW, Rhee SG. Isoforms of mammalian peroxiredoxin that reduce peroxides in presence of thioredoxin. Methods Enzymol. 1999; 300:219–226. PMID: 9919524.

4. Seo MS, Kang SW, Kim K, Baines IC, Lee TH, Rhee SG. Identification of a new type of mammalian peroxiredoxin that forms an intramolecular disulfide as a reaction intermediate. J Biol Chem. 2000; 275(27):20346–20354. PMID: 10751410.

5. Kang SW, Chae HZ, Seo MS, Kim K, Baines IC, Rhee SG. Mammalian peroxiredoxin isoforms can reduce hydrogen peroxide generated in response to growth factors and tumor necrosis factor-α. J Biol Chem. 1998; 273(11):6297–6302. PMID: 9497357.

6. Okado-Matsumoto A, Matsumoto A, Fujii J, Taniguchi N. Peroxiredoxin IV is a secretable protein with heparin-binding properties under reduced conditions. J Biochem. 2000; 127(3):493–501. PMID: 10731722.

7. Krapfenbauer K, Engidawork E, Cairns N, Fountoulakis M, Lubec G. Aberrant expression of peroxiredoxin subtypes in neurodegenerative disorders. Brain Res. 2003; 967(1-2):152–160. PMID: 12650976.

8. Cumming RC, Dargusch R, Fischer WH, Schubert D. Increase in expression levels and resistance to sulfhydryl oxidation of peroxiredoxin isoforms in amyloid beta-resistant nerve cells. J Biol Chem. 2007; 282(42):30523–30534. PMID: 17761673.
9. Yu G, Nishimura M, Arawaka S, Levitan D, Zhang L, Tandon A, Song YQ, Rogaeva E, Chen F, Kawarai T, Supala A, Levesque L, Yu H, Yang DS, Holmes E, Milman P, Liang Y, Zhang DM, Xu DH, Sato C, Rogaev E, Smith M, Janus C, Zhang Y, Aebersold R, Farrer LS, Sorbi S, Bruni A, Fraser P, St George-Hyslop P. Nicastrin modulates presenilin-mediated notch/glp-1 signal transduction and βAPP processing. Nature. 2000; 407(6800):48–54. PMID: 10993067.
10. Li YM. Gamma-secretase: a catalyst of Alzheimer disease and signal transduction. Mol Interv. 2001; 1(4):198–207. PMID: 14993342.
11. Nam SH, Seo SJ, Goo JS, Kim JE, Choi SI, Lee HR, Hwang IS, Jee SW, Lee SH, Bae CJ, Park JY, Kim HS, Shim SB, Hwang DY. Pen-2 overexpression induces Aβ-42 production, memory defect, motor activity enhancement and feeding behavior dysfunction in NSE/Pen-2 transgenic mice. Int J Mol Med. 2011; 28:961–971. PMID: 21822534.

12. Hwang DY, Chae KR, Kang TS, Hwang JH, Lim CH, Kang HK, Goo JS, Lee MR, Lim HJ, Min SH, Cho JY, Hong JT, Song CW, Paik SG, Cho JS, Kim YK. Alterations in behavior, amyloid beta-42, caspase-3, and Cox-2 in mutant PS2 transgenic mouse model of Alzheimer's disease. FASEB J. 2002; 16:805–813. PMID: 12039862.
13. Prajapati KD, Sharma SS, Roy N. Upregulation of albumin expression in focal ischemic rat brain. Brain Res. 2010; 1327:118–124. PMID: 20193666.

14. Kim HJ, Chae HZ, Kim YJ, Kim YH, Hwangs TS, Park EM, Park YM. Preferential elevation of Prx I and Trx expression in lung cancer cells following hypoxia and in human lung cancer tissues. Cell Biol Toxicol. 2003; 19(5):285–298. PMID: 14703116.

15. Chang JW, Lee SH, Jeong JY, Chae HZ, Kim YC, Park ZY, Yoo YJ. Peroxiredoxin-I is an autoimmunogenic tumor antigen in non-small cell lung cancer. FEBS Lett. 2005; 579(13):2873–2877. PMID: 15876430.

16. Nonn L, Berggren M, Powis G. Increased expression of mitochondrial peroxiredoxin-3 (thioredoxin peroxidase-2) protects cancer cells against hypoxia and drug-induced hydrogen peroxide-dependent apoptosis. Mol Cancer Res. 2003; 1(9):682–689. PMID: 12861054.
17. Oda A, Tamaoka A, Araki W. Oxidative stress up-regulates presenilin 1 in lipid rafts in neuronal cells. J Neurosci Res. 2010; 88(5):1137–1145. PMID: 19885829.

18. Yao J, Taylor M, Davey F, Ren Y, Aiton J, Coote P, Fang F, Chen JX, Yan SD, Gunn-Moore FJ. Interaction of amyloid binding alcohol dehydrogenase/Abeta mediates up-regulation of peroxiredoxin II in the brains of Alzheimer's disease patients and a transgenic Alzheimer's disease mouse model. Mol Cell Neurosci. 2007; 35(2):377–382. PMID: 17490890.
Figure 1
Pen-2 expression and accumulation of Aβ-42 peptides in the brain of CMV/hPen-2 Tg mice. (A) Brain specific expression of the hPen-2 transgenes in the Tg mice by RT-PCR. The β-actin signal was used as the control, and the transcript (640-bp) indicates RNA loading. In addition, the RT-PCR products for hPen-2 (284-bp) are indicated. The density of the amplified transcripts was quantified. (B) Immunostaining analysis of the Aβ-42 peptides. The brains were taken from NSE/hPen-2 Tg mice and Non-Tg mice after perfusion. The level of the Aβ-42 peptide was detected in the immunostaining analysis using an Aβ-42 specific antibody. A high intensity was observed in the hippocampus of the NSE/hPen-2 Tg mice when compared with the Non-Tg mice at 200× magnification. The data represents the mean±SD from three replicates. *P<0.05; significant difference between NSE/hPen-2 Tg and Non-Tg mice.
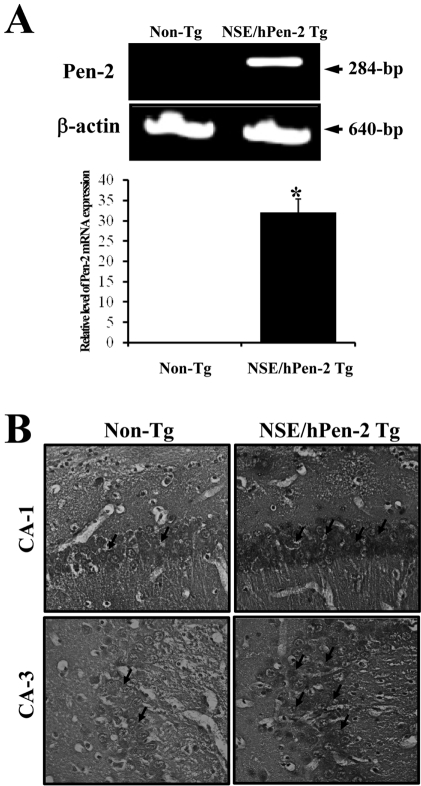
Figure 2
Expression of Prx I and VI mRNA and protein in the brain of NSE/hPen-2 Tg and Non-Tg mice. (A and B) Total RNA was purified from the whole brain of both mice, and the gene expression of the Prx I and VI were detected by RT-PCR. The β-actin signal was used as the control, and the transcript (640-bp) indicates the RNA loading. (C and D) Total tissue lysates were prepared from the brain tissue of NSE/hPen-2 Tg and Non-Tg mice as described in the Materials and Methods sections. Fifty micrograms of protein per sample were immunoblotted using antibodies for each protein. Three samples were assayed in triplicate via Western blotting. The values are expressed as the means±SD. *P<0.05; significant difference between NSE/hPen-2 Tg and Non-Tg mice.
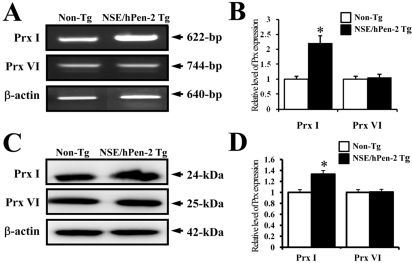
Figure 3
Effects of the overexpression of Prx I on the component expression of γ-secretase complex in SH-SY5Y cells. (A) Construction of CMV/hPrx I plasmid. The pCMV/hPrx I harbors the cDNA encoding hPrx I under the control of the CMV gene promoter. (B and C) Total cell lysates were prepared from SH-SY5Y cells transfected with CMV control vector and CMV/hPrx I vector as described in the Materials and Methods section. Fifty micrograms of protein per sample were immunoblotted using antibodies for each protein. Three samples were assayed in triplicate via Western blotting. The values were expressed as means±SD. *P<0.05 is the significance level compared to the vehicle-treated group.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download