Abstract
Background
Currently, no generally accepted laboratory marker for monitorizing the disease activity and therapy response of psoriasis is known.
Objective
The aim of the study is to evaluate the effects of systemic therapies on C-reactive protein (CRP) and the neutrophil-lymphocyte ratio (NLR) in psoriasis.
Methods
One hundred patients with psoriasis treated with narrow band ultraviolet B, acitretin, cyclosporine, methotrexate, adalimumab, etanercept, and ustekinumab were prospectively evaluated. At baseline and at week 12, CRP, NLR, and Psoriasis Area and Severity Index (PASI) were evaluated.
Results
A statistically significant decrease was observed in PASI scores, CRP, and NLR values from the baseline to the 12-week visit (p=0.001, p=0.001, p=0.001, respectively). The reduction in PASI scores and NLR values was positively correlated (r=0.460, p=0.001). The comparisons between treatment groups revealed that the median decrease in NLR values was statistically higher in the adalimumab group than in the methotrexate group (p=0.007). And the median decrease in PASI scores was significantly higher in the adalimumab group compared with the methotrexate and acitretin therapy group (p=0.007, p=0.042, respectively).
Psoriasis is a common, chronic, inflammatory and immuneediated skin disease associated with cardiovascular comorbidities1.
The Psoriasis Area and Severity Index (PASI) is the currently preferred tool to assess disease severity and to evaluate the efficacy of the treatment2. Since the method is limited by physician-dependent scoring, many laboratory markers have been studied in psoriasis to find an objective tool to assess disease activity3456789101112.
The levels of serum C-reactive protein (CRP) and neutrophil-lymphocyte ratio (NLR), both of which are inexpensive and easily accessible markers, have been reported to be elevated in patients with psoriasis, and in some studies, CRP and NLR were found to be correlated with the disease activity regarding as PASI891011. Moreover, elevated levels of both CRP and NLR have been demonstrated to be related with cardiovascular diseases (CVD) and with cardiovascular comorbidities in psoriasis910111213.
In the search for objective assessment tools for psoriasis, CRP and NLR have been used to demonstrate treatment efficacy in psoriasis. But the few studies on this topic have mostly been performed among patients receiving biologic agents314151617.
The present study sought to evaluate the levels of CRP and NLR in patients with psoriasis under different systemic treatment modalities, including narrow-band ultraviolet B (NBVB), acitretin, methotrexate (MTX), cyclosporine, adalimumab (ADA), etanercept (ETA), and ustekinumab (UST).
A single-centre observational study was performed. One hundred outpatients diagnosed with chronic plaque type psoriasis by a clinical or histopathological examination were recruited from the Department of Dermatology.
Data on the baseline demographics, clinical characteristics (including information about the duration of psoriasis, medical history, tobacco smoking and alcohol habits), concomitant arthritis and/or nail involvement were recorded. The inclusion criteria for patients were as follows: 1) patients with chronic plaque type psoriasis, 2) aged greater than 18 years, 3) who had not received any topical and/or systemic psoriasis treatment prior to 6 months, and 4) who had a clinical indication to initiate systemic therapy for psoriasis according to the European psoriasis treatment guideline18.
The exclusion criteria included a lack of co-operability, any contraindication for the treatment, any significant infection, immunodeficiency, pregnancy or breastfeeding, the use of systemic and/or topical treatment during the last 6 months before the inclusion, and malignancy.
The appropriate treatment modality was decided upon together with the patient, according to the psoriasis treatment guidelines18 and the patients' characteristics. Each patient was started on one of the following psoriasis treatment: NB-UVB, acitretin, cyclosporine, MTX, ADA, ETA or UST. Before and during the treatment, each patient underwent laboratory and physical controls, as recommended in the treatment guidelines18.
The treatment regimes were as follows: ETA 50 mg subcutaneous (SC) injection once a week, an initial dose of ADA 80 mg and 40 mg SC injection every other week thereafter. UST was administrated SC (45 mg) at weeks 0, 4, and every 12 weeks thereafter. MTX doses were 15±25 mg subcutaneously once a week, cyclosporine was administered orally every day (2.5~5 mg/kg), acitretin was administered orally (10~25 mg/day), and NB-UVB (7002k Cabinet; Waldmann, Villingen-Schwenningen, Germany) was administered three times a week with a gradual increase in dosing.
None of the patients received any topical treatments other than moisturizers.
The serum CRP and complete blood count were measured at the baseline/before treatment and at 12 weeks of treatment.
Psoriasis was graded according to the PASI calculated at the baseline and at 12 weeks of treatment by a singlelinded dermatologist.
The study was reviewed and approved by Şişli Hamidiye Etfal Training and Research Hospital Clinical Research Ethics Committee (IRB no. 574, 10/01/2016), and all the individuals provided written informed consent. The study was carried out according to the principles expressed in the Declaration of Helsinki.
The Number Cruncher Statistical System 2007 (Kaysville, UT, USA) program was used for the statistical analysis. The descriptive data were expressed with mean± tandard deviation, numeric variables and percentages. In the analysis of normally distributed variables, a Student's t-test was applied to examine the differences between the two groups. The differences between the two independent groups were examined using the non-parametric Mann–Whitney U-test for non-normally distributed variables. The comparisons between three or more groups of non-normally distributed were evaluated with a Kruskal–Wallis test and Wilcoxon Student's t-test using Bonferroni's correction for multiple comparisons. The intragroup comparisons of normally distributed data were performed with paired samples test, whereas the non-normally distributed data of intragroup comparisons were evaluated with a Wilcoxon signed ranks test. A Pearson χ2 test was used to compare qualitative variables. The correlation analysis was performed by calculating Pearson and Spearman's rank correlation, and p<0.05 was considered statistically significant.
A total of 100 patients (39 female and 61 male) with chronic plaque type psoriasis were included in the study. The mean age of the patients was 40.80±13.52 years. The duration of the disease ranged from 6 to 516 months (mean 150.29±106.40).
The prevalence of an accompanying disease was 14.0%, and hypertension (n=6), diabetes (n=3) and hyperlipidaemia (n=3) were the most common diseases; 60.0% of the patients were current tobacco smokers, while 28.0% of the patients regularly consume alcohol.
Nail involvement was noted in 35.0% (n=35) of the patients, while 13.0% (n=13) of the patients had concomitant psoriatic arthritis.
The mean PASI score of the patients at the baseline was 15.08±8.81.
The mean CRP concentrations was 5.09±3.37 at screening, while the mean NLR values was 2.34±1.06 at the screening.
The baseline patient demographics and disease characteristics are reported in Table 1.
All the patients received one of the following therapies: ADA (n=19), acitretin (n=11), NB-UVB (n=7), ETA (n=2), MTX (n=30), cyclosporine (n=12), or UST (n= 9).
No serious adverse events requiring treatment cessation occurred throughout the study.
We investigated the correlation between patients' characteristics and PASI, CRP, and NLR values (Student's t-test, Mann–Whitney U-test).
A positive correlation was seen between the PASI score and the values of CRP and NLR at the baseline (r=0.349, p=0.001; r=0.271, p=0.006, respectively). Also, at screening, CRP and NLR values were positively correlated (r=0.376, p=0.001; Spearman's correlation coefficient) (Table 2).
No correlations were found between the baseline values of CRP, NLR, and PASI scores and disease duration p>0.05; Spearman's correlation coefficient).
A positive correlation was found between the age and CRP levels at the baseline (r=0.198, p=0.049). Similarly, the age of the patients was also correlated with NLR values at screening (r=0.312, p=0.002; Spearman's correlation coefficient).
When the values of PASI, NLR, and CRP at screening was evaluated in terms of sex, male patients were shown to have statistically higher NLR values than female (p=0.001; Mann–Whitney U-test).
No association was observed between smoking, alcohol consumption, accompanying diseases, nail involvement or concomitant arthritis and the CRP, NLR, and PASI scores p>0.05, for all).
Changes in serum CRP, NLR, and PASI scores were examined before and after the treatment.
The decreases in the PASI scores and CRP and NLR values from the baseline to the 12th-week visit were statistically significant (p=0.001, p=0.001, and p=0.001, respectively; paired samples test, Wilcoxon signed ranks test; Fig. 2). The relationship between the changes in the NLR and CRP values and PASI scores and patients' characteristics including gender, smoking habbit, accompanying disease, etc. are shown in Table 4 (Mann–Whitney U-test).
Clinical improvement was reflected in the improved PASI scores. The median reduction in PASI scores from the baseline to week 12 was 12.56±8.26, which was statistically significant (p=0.001; paired samples test).
The decrease in the levels of CRP from baseline to week 12 was 1.97±3.15, which was also statistically significant (p=0.001; p<0.01; Wilcoxon signed ranks test).
The mean reduction in NLR values from the baseline to week 12 was 0.53±0.96, which was statistically significant (p=0.001; Wilcoxon signed ranks test).
A positive correlation was observed between the PASI and CRP values at week 12 (r=0.226, p=0.024). No correlation was found between the PASI and NLR values at week 12 p>0.05). The NLR and CRP values were also not correlated at week 12 p>0.05; Spearman's correlation coefficient) (Table 2).
The reduction in the PASI scores and NLR values was positively correlated (r=0.460, p=0.001). The decrease in the NLR and CRP levels was also positively correlated (r=0.227, p=0.023). However, the decrease in the scores of PASI and CRP from the baseline to the 12-week visit was not correlated p>0.05; Spearman's correlation coefficient) (Table 5).
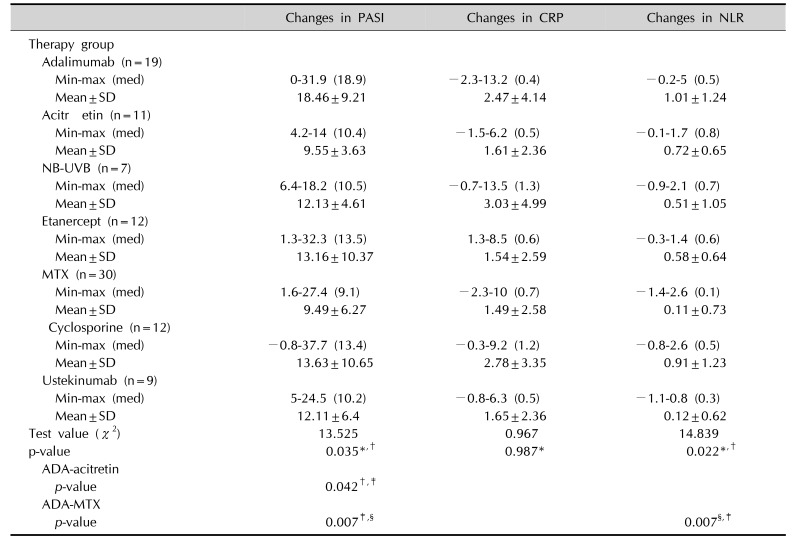
The decrease in the CRP, NLR, and PASI scores were evaluated in each treatment group (Kruskal–Wallis test).
Mean reductions in PASI scores were greatest in the ADA group, followed by cyclosporine, ETA, NB-UVB, UST, acitretin, and MTX.
Mean reductions in CRP levels were greatest in the NB-UVB group, followed by cyclosporine, ADA, UST, acitretin, ETA and MTX.
Mean reductions in NLR values were greatest in the ADA group, followed by cyclosporine, acitretin, ETA, NB-UVB, UST, and MTX.
When the reductions in the PASI scores were evaluated between subgroups of treatment, the reduction in the PASI scores was significantly higher in the ADA group compared with the MTX and acitretin group (p=0.007, p=0.042, respectively). In the subgroup comparisons, the decrease in NLR was statistically higher in the ADA group than in the MTX group (p=0.007; Fig. 3, 4).
The decrease in the CRP levels was significant in the study group, but no difference was observed between the subgroups of treatment (p>0.05; Table 6).
Several researchers have investigated the levels of inflammatory parameters in psoriasis and the association between the biomarkers and disease activity. Among many parameters, we chose to evaluate the levels of CRP and NLR since they are both reliable parameters indicating systemic inflammation and cardiovascular risks, as well easily accessible and inexpensive methods.
Psoriasis and CVD based on atherogenesis both share the immunoinflammatory mechanisms that provide the link between the two diseases19. It has been suggested that systemic anti-inflammatory therapy might be beneficial in reducing the CVD risk among patients with psoriasis1920. In this study, we prospectively analysed the effects of systemic treatments on inflammatory parameters in psoriasis over a period of 12 weeks.
At the baseline evaluation prior to any treatment, the PASI scores were positively correlated with the CRP and NLR values. Moreover, CRP and NLR were also positively correlated. Since, PASI is an established tool in scoring disease severity, these findings may lead us to the suggestion that CRP and NLR might be objective laboratory markers indicating disease severity in psoriasis. Previous reports are in line with this hypothesis89101112131415.
At week 12, a positive correlation between the PASI and CRP values was noted, but no correlation was found between CRP and NLR or between PASI and NLR.
The present study revealed a statistically significant decrease in the values of PASI, serum CRP, and NLR in the study group who received different therapies, including acitretin, MTX, cyclosporine, ADA, ETA, UST, and NB-UVB for a period of 12 weeks.
When we evaluated the changes in CRP and NLR according to patients' characteristics, we found that the decrease in NLR values was statistically higher in males than in females. The higher NLR values in males at the baseline may explain this. Still, it cannot be ruled out that a different gender-based response to the treatment may be present.
Median reductions in PASI were greater in non-smokers than in patients who are smokers, which may lead to the suggestion that smoker psoriasis patients may not respond to therapy as well as non-smokers. Smoking has already been shown to be related to the severity of psoriasis2122, but data on the impact of smoking on therapy response is insufficient. In a recent retrospective study, no statistical difference in terms of clinical improvement in psoriasis among non-smokers and smokers on various systemic treatment regimens was found23. But if larger epidemiologic studies confirm this relationship, strict guidelines for smokers with psoriasis may be implemented.
The reductions in CRP levels were significantly lower in psoriasis patients who had an accompanying disease than in those who did not. We believe that the ongoing inflammation due to accompanying disease may lead to late or low-response levels of CRP.
Interestingly, although it is known that nail involvement and the occurrence of psoriatic arthritis is related to severe inflammation, no impact was seen in terms of parameter changes24.
Treatment success, defined as a decrease in the PASI score, was paralleled by reduced serum levels of NLR, but not with CRP. The correspondence between decreases in the NLR levels and PASI scores suggests that NLR and psoriasis severity may be linked. The reduction in CRP levels and NLR was also correlated.
The main purpose of the study was to investigate whether any of the treatment options for psoriasis provide a better anti-inflammatory effect, demonstrated as reductions in CRP and NLR values. Beyond that, we wanted to emphasize that the anti-inflammatory effect could prevent cardiovascular events in patients with psoriasis. In line with our study, some other studies have been performed, but the results of these studies evaluating the cardiovascular events in psoriasis patients under anti-inflammatory treatment have been conflictory202526. Fewer reports have investigated the changes in laboratory markers under sytemic therapy, and they have been mostly conducted among psoriasis patients recieving the tumor necrosis factor (TNF) blocker or MTX therapy. In different studies, significant reductions in PASI, CRP, interleukin-6, TNF-α, resistin and lipoprotein and an increase in adiponectin were found in patients under various systemic treatments3471527. Montaudié et al.28 observed a higher decrease in CRP levels in patients in a biologic agent therapy (infliximab, ETA, ADA, and UST) group than in a conventional systemic therapy (MTX, cyclosporine, and acitretin) group. They found no correlation between the reduction in CRP, erithrocyte sedimentation rate, HbA1c, and PASI28.
In a distinct departure from the literature, we wanted to evaluate the effects of several therapy agents used in psoriasis on CRP and NLR which are indicators of CVD and systemic inflammation.
Concerning the changes in CRP levels and NLR and PASI scores after treatment, we found a statistically significant reduction in these values regardless of the type of treatment.
However, when analyses between two groups were performed, these differences had only two results of statistical significance: (1) the decrease in NLR values was statistically higher in ADA group compared with the MTX group, (2) the reduction in PASI scores was statistically higher in the ADA group than in the MTX and acitretin groups.
It is also noteworthy that although statistically significant decreases in the PASI, CRP, and NLR values were demonstrated in patients receiving MTX therapy in the present study, the MTX group had the least decreases in these values compared with other agents.
However, this trend must be viewed with caution because of the small number of patients in each treatment group, which makes the study unpowered. The short-term follow-up period of the study might be another explanation. During the follow-up period, none of the patients had any changes in their lives including, habits (smoking, alcohol, etc.), medical events, dietary habits, any treatment, sports, social activities, etc. Since this information is based on the declarations of the patients, it may be assumed as a limitation. The control group of the study did not consist of healthy subjects. Patients with psoriasis who are not receiving therapy were included as control group and after receiving psoriasis therapy for 12 weeks they presented the study group.
We believe that the results of the study should be acknowledged for several aspects. First of all, this is the first study examining the impact of so many treatment options in psoriasis on CRP and NLR. Moreover, both CRP and NLR are easily accessed parameters in daily practice that may be used to assess the disease activity and to evaluate the efficacy of treatment. And most importantly, since they are both markers of CVD, they may be used to determine the optimal therapy for psoriasis according to a patient's characteristics, preventing cardiovascular comorbidities.
We suggest that controlling the systemic inflammation in psoriasis may prevent cardiovascular comorbidities, which are known to be associated with severe psoriasis. Further studies addressing the impact of long-term systemic therapies on cardiovascular functions in patients with psoriasis are warranted.
References
1. Biljan D, Situm M, Kostović K, Batinac T, Matisić D. Acute phase proteins in psoriasis. Coll Antropol. 2009; 33:83–86. PMID: 19408608.
2. Langley RG, Ellis CN. Evaluating psoriasis with Psoriasis Area and Severity Index, Psoriasis Global Assessment, and Lattice System Physician's Global Assessment. J Am Acad Dermatol. 2004; 51:563–569. PMID: 15389191.

3. Coimbra S, Oliveira H, Reis F, Belo L, Rocha S, Quintanilha A, et al. C-reactive protein and leucocyte activation in psoriasis vulgaris according to severity and therapy. J Eur Acad Dermatol Venereol. 2010; 24:789–796. PMID: 20002653.

4. Coimbra S, Oliveira H, Reis F, Belo L, Rocha S, Quintanilha A, et al. Circulating adipokine levels in Portuguese patients with psoriasis vulgaris according to body mass index, severity and therapy. J Eur Acad Dermatol Venereol. 2010; 24:1386–1394. PMID: 20337818.

5. Ctirad A, Lenka B, David P, Zdenek F, Kveta H, Karel E, et al. Goeckerman's therapy for psoriasis with special reference to serum pentraxin 3 level. Int J Dermatol. 2008; 47:1011–1014. PMID: 18986345.

6. Chodorowska G, Wojnowska D, Juszkiewicz-Borowiec M. C-reactive protein and alpha2-macroglobulin plasma activity in medium-severe and severe psoriasis. J Eur Acad Dermatol Venereol. 2004; 18:180–183. PMID: 15009298.

7. Coimbra S, Oliveira H, Reis F, Belo L, Rocha S, Quintanilha A, et al. Interleukin (IL)-22, IL-17, IL-23, IL-8, vascular endothelial growth factor and tumour necrosis factor-α levels in patients with psoriasis before, during and after psoralenultraviolet A and narrowband ultraviolet B therapy. Br J Dermatol. 2010; 163:1282–1290. PMID: 20716219.

8. Sen BB, Rifaioglu EN, Ekiz O, Inan MU, Sen T, Sen N. Neutrophil to lymphocyte ratio as a measure of systemic inflammation in psoriasis. Cutan Ocul Toxicol. 2014; 33:223–227. PMID: 24147939.

9. Yurtdaş M, Yaylali YT, Kaya Y, Ozdemir M, Ozkan I, Aladağ N. Neutrophil-to-lymphocyte ratio may predict subclinical atherosclerosis in patients with psoriasis. Echocardiography. 2014; 31:1095–1104. PMID: 24447343.

10. Ataseven A, Bilgin AU, Kurtipek GS. The importance of neutrophil lymphocyte ratio in patients with psoriasis. Mater Sociomed. 2014; 26:231–233. PMID: 25395882.

11. Kim DS, Shin D, Lee MS, Kim HJ, Kim DY, Kim SM, et al. Assessments of neutrophil to lymphocyte ratio and platelet to lymphocyte ratio in Korean patients with psoriasis vulgaris and psoriatic arthritis. J Dermatol. 2016; 43:305–310. PMID: 26381893.

12. Polat M, Bugdayci G, Kaya H, Oğuzman H. Evaluation of neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio in Turkish patients with chronic plaque psoriasis. Acta Dermatovenerol Alp Pannonica Adriat. 2017; 26:97–100. PMID: 29264899.

13. Gerkowicz A, Pietrzak A, Szepietowski JC, Radej S, Chodorowska G. Biochemical markers of psoriasis as a metabolic disease. Folia Histochem Cytobiol. 2012; 50:155–170. PMID: 22763973.

14. Strober BE, Poulin Y, Teller C, Wang Y, Williams DA, Goldblum OM. Changes in C-reactive protein in patients with moderate-to-severe psoriasis switched to adalimumab therapy after suboptimal response to etanercept, methotrexate or phototherapy. J Eur Acad Dermatol Venereol. 2014; 28:1701–1706. PMID: 24422992.

15. Strober B, Teller C, Yamauchi P, Miller JL, Hooper M, Yang YC, et al. Effects of etanercept on C-reactive protein levels in psoriasis and psoriatic arthritis. Br J Dermatol. 2008; 159:322–330. PMID: 18503600.

16. Zhang L, Wiles C, Martinez LR, Han G. Neutrophil-to-lymphocyte ratio decreases after treatment of psoriasis with therapeutic antibodies. J Eur Acad Dermatol Venereol. 2017; 31:e491–e492. PMID: 28502119.

17. Asahina A, Kubo N, Umezawa Y, Honda H, Yanaba K, Nakagawa H. Neutrophil-lymphocyte ratio, platelet-lymphocyte ratio and mean platelet volume in Japanese patients with psoriasis and psoriatic arthritis: response to therapy with biologics. J Dermatol. 2017; 44:1112–1121. PMID: 28493493.

18. Nast A, Gisondi P, Ormerod AD, Saiag P, Smith C, Spuls PI, et al. European S3-Guidelines on the systemic treatment of psoriasis vulgaris--update 2015--short version--EDF in cooperation with EADV and IPC. J Eur Acad Dermatol Venereol. 2015; 29:2277–2294. PMID: 26481193.
19. Alexandroff AB, Pauriah M, Camp RD, Lang CC, Struthers AD, Armstrong DJ. More than skin deep: atherosclerosis as a systemic manifestation of psoriasis. Br J Dermatol. 2009; 161:1–7.

20. Ahlehoff O, Skov L, Gislason G, Lindhardsen J, Kristensen SL, Iversen L, et al. Cardiovascular disease event rates in patients with severe psoriasis treated with systemic antiinflammatory drugs: a Danish real-world cohort study. J Intern Med. 2013; 273:197–204. PMID: 22963528.

21. Adışen E, Uzun S, Erduran F, Gürer MA. Prevalence of smoking, alcohol consumption and metabolic syndrome in patients with psoriasis. An Bras Dermatol. 2018; 93:205–211.

22. Fortes C, Mastroeni S, Leffondré K, Sampogna F, Melchi F, Mazzotti E, et al. Relationship between smoking and the clinical severity of psoriasis. Arch Dermatol. 2005; 141:1580–1584. PMID: 16365261.

23. Kinahan CE, Mazloom S, Fernandez AP. Impact of smoking on response to systemic treatment in patients with psoriasis: a retrospective case-control study. Br J Dermatol. 2015; 172:428–436. PMID: 25142556.

24. Daulatabad D, Grover C, Kashyap B, Dhawan AK, Singal A, Kaur IR. Clinical and serological characteristics of nail psoriasis in Indian patients: a cross-sectional study. Indian J Dermatol Venereol Leprol. 2017; 83:650–655. PMID: 28656915.

25. Abuabara K, Lee H, Kimball AB. The effect of systemic psoriasis therapies on the incidence of myocardial infarction: a cohort study. Br J Dermatol. 2011; 165:1066–1073. PMID: 21777216.

26. Boehncke S, Salgo R, Garbaraviciene J, Beschmann H, Hardt K, Diehl S, et al. Effective continuous systemic therapy of severe plaque-type psoriasis is accompanied by amelioration of biomarkers of cardiovascular risk: results of a prospective longitudinal observational study. J Eur Acad Dermatol Venereol. 2011; 25:1187–1193. PMID: 21241371.

27. Coimbra S, Oliveira H, Reis F, Belo L, Rocha S, Quintanilha A, et al. Psoriasis therapy and cardiovascular risk factors: a 12-week follow-up study. Am J Clin Dermatol. 2010; 11:423–432. PMID: 20429617.
28. Montaudié H, Albert-Sabonnadière C, Acquacalda E, Fontas E, Danré A, Roux C, et al. Impact of systemic treatment of psoriasis on inflammatory parameters and markers of comorbidities and cardiovascular risk: results of a prospective longitudinal observational study. J Eur Acad Dermatol Venereol. 2014; 28:1186–1191. PMID: 23981008.

Fig. 1
Correlations between PASI scores and the values of CRP and NLR at baseline. PASI: Psoriasis Area and Severity Index, CRP: C-reactive protein, NLR: neutrophil-lymphocyte ratio.
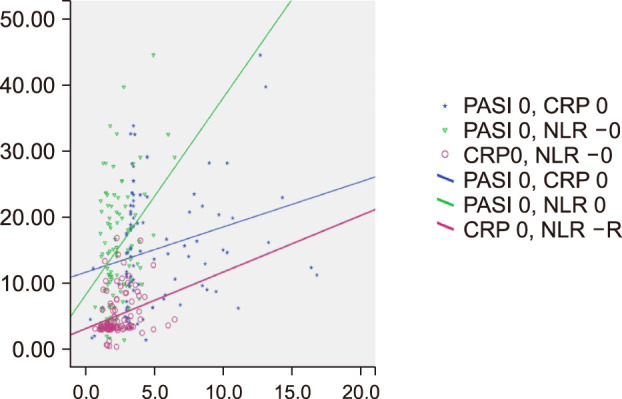
Fig. 2
Values of PASI, CRP, and NLR at baseline and week 12. PASI: Psoriasis Area and Severity Index, CRP: C-reactive protein, NLR: neutrophil-lymphocyte ratio.
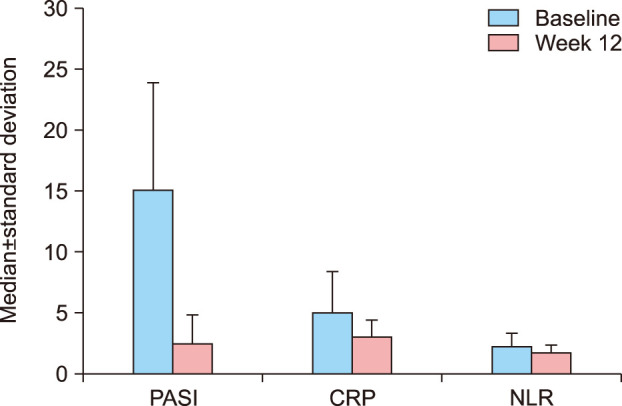
Fig. 3
Changes in PASI scores in therapy subgroups. PASI: Psoriasis Area and Severity Index, ADA: adalimumab, NB-UVB: narrow-band ultraviolet B, ETA: etanercept, MTX: methotrexate, UST: ustekinumab.
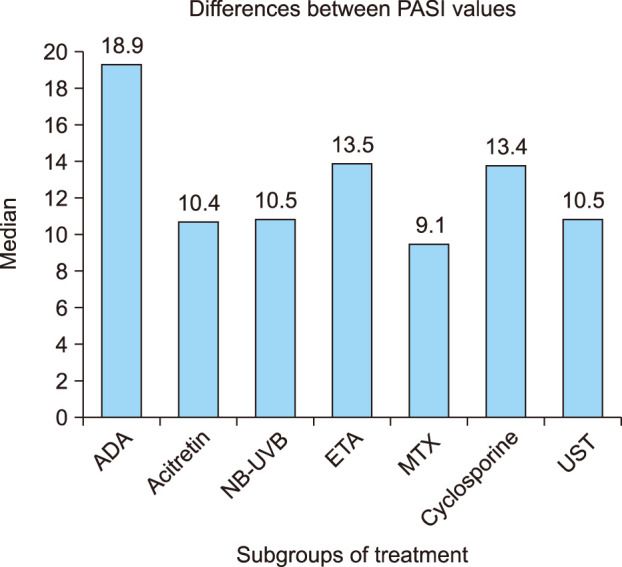
Fig. 4
Changes in NLR values in therapy subgroups. NLR: neutrophil-lymphocyte ratio, ADA: adalimumab, NB-UVB: narrowband ultraviolet B, ETA: etanercept, MTX: methotrexate, UST: ustekinumab.
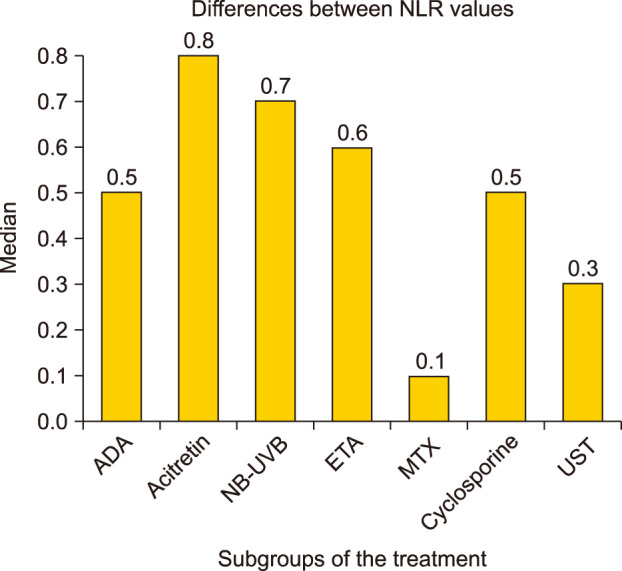
Table 1
Demographic features and the disease characteristics of the patients

Table 2
Relationship between the values of CRP, NLR, and PASI at baseline and at 12-week
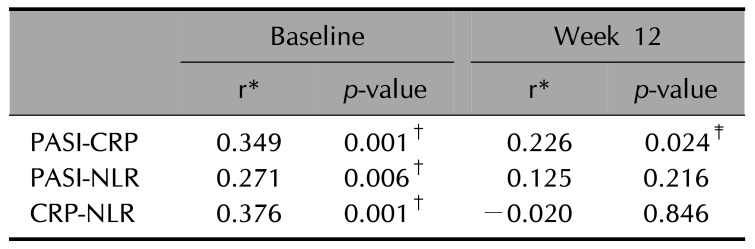
| Baseline | Week 12 | |||
|---|---|---|---|---|
| r* | p-value | r* | p-value | |
| PASI-CRP | 0.349 | 0.001† | 0.226 | 0.024‡ |
| PASI-NLR | 0.271 | 0.006† | 0.125 | 0.216 |
| CRP-NLR | 0.376 | 0.001† | −0.020 | 0.846 |
Table 3
CRP, NLR, and PASI values at baseline and week 12, changes in CRP, NLR, and PASI values
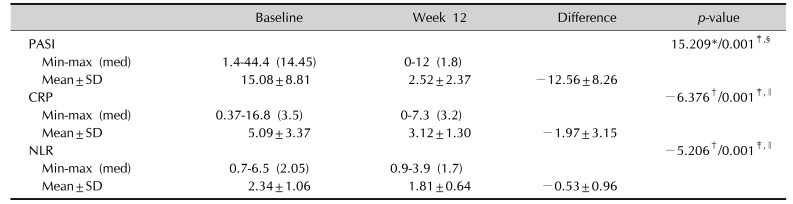
Table 4
Relationship between the changes in the values of PASI, CRP, NLR, and patients' characteristics
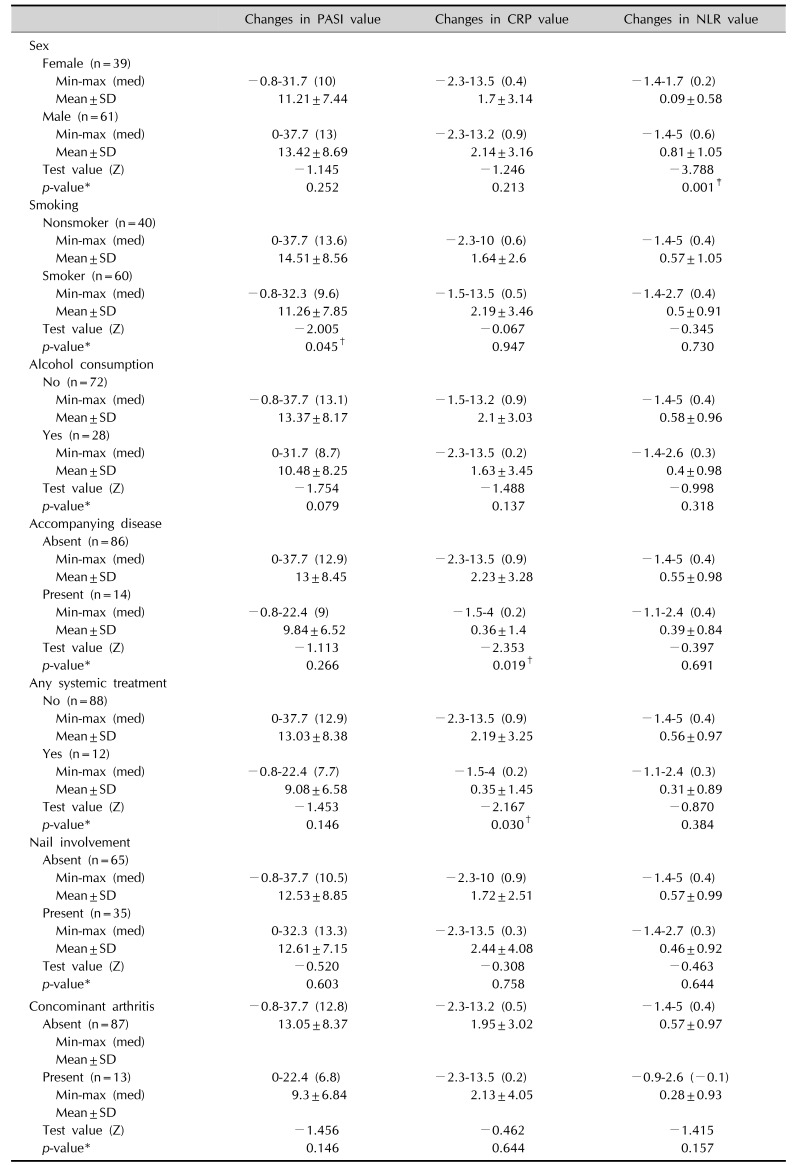
Table 5
Relationship between the changes in values of PASI, CRP, and NLR between baseline and at 12-week
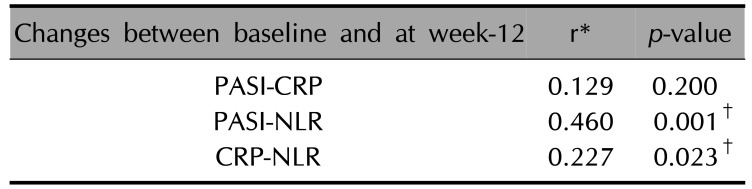
| Changes between baseline and at week-12 | r* | p-value |
|---|---|---|
| PASI-CRP | 0.129 | 0.200 |
| PASI-NLR | 0.460 | 0.001† |
| CRP-NLR | 0.227 | 0.023† |
Table 6
Changes in CRP, NLR, and PASI between baseline and week 12 among therapy subgroups




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download