INTRODUCTION
METHODS
Reagents
Cell culture
Adenovirus infection
Measurement of ROS
Permeability of the BBB in vitro
Determination of trans-endothelial electrical resistance
Western blotting
Measurement of NAD(P)H oxidase activity
Statistical analysis
RESULTS
LPS disrupted BBB integrity
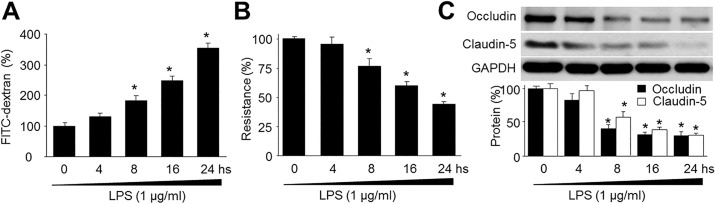 | Fig. 1LPS decreases blood-brain barrier integrity and reduces the expression of tight-junction proteins in human brain microvascular endothelial cells (HBMECs).(A) Confluent monolayer of HBMECs was cultured on Transwell filters and the diffusion of FITC-conjugated dextran (150 kDa; 100 µg/ml) in LPS (1 µg/ml) was measured at various time points. Control was set up as 100%. Data are expressed as the mean±SEM. N is 5 in each group. *p<0.05 vs control. (B) Trans-endothelial electrical resistance was measured in real time in confluent monolayer of HBMECs. Control was set up as 100%. Data are expressed as the mean±SEM. N is 5 in each group. *p<0.05 vs control. (C) Representative Western blot of tight-junction proteins (occludin and claudin-5). The blot is representative of three blots from three independent experiments. *p<0.05 vs control.
|
LPS reduced expressions of tight-junction proteins
LPS increased production of superoxide anions
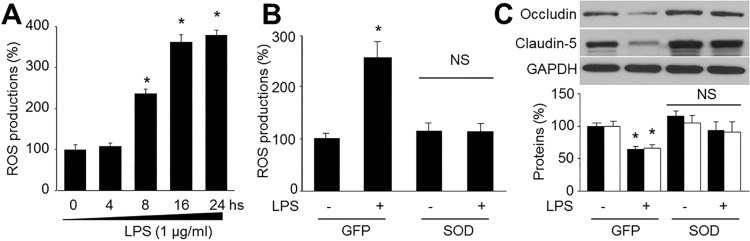 | Fig. 2Reactive oxygen species (ROS) mediates LPS-reduced tight-junction protein expressions in human brain microvascular endothelial cells (HBMECs).(A) Confluent monolayer of HBMECs were cultured in LPS (1 µg/ml) was measured at various time points. ROS productions were assayed by DHE fluorescence. Data are expressed as the mean±SEM. N is 5 in each group. *p<0.05 vs control. (B and C) Cultured HBMECs were infected with adenovirus containing superoxide dismutase (SOD) for 48 hours and then exposure to LPS of 1 µg/ml for 24 hours. (B) Superoxide anion productions and (C) levels of occludin and claudin-5 expression were measured. N is 5 in each group. *p<0.05 vs control.
|
Overexpression of SOD prevented LPS-induced reduction of tight-junction proteins
Inhibition of NAD(P)H oxidase attenuated LPS-induced reduction of tight-junction proteins
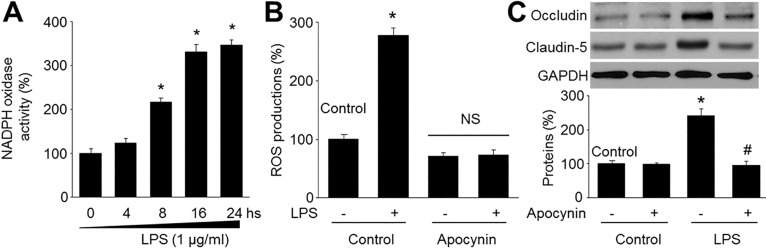 | Fig. 3LPS decreases tight-junction protein levels via upregulation of NAD(P)H oxidase in microvascular endothelial cells.(A) Confluent monolayer of HBMECs were cultured in LPS (1 µg/ml) was measured at various time points. NAD(P)H oxidase activity was was assayed by DHE fluorescence. Data are expressed as the mean±SEM. N is 5 in each group. *p<0.05 vs control. (B and C) Cultured HBMECs were pretreated with apocynin (10 µM) for 30 mins and then co-incubated with LPS (1 µg/ml) for 24 hours. (B) ROS productions were detected by DHE. N is 5 in each group. *p<0.05 vs control. NS indicates no significance. (C) Levels of occludin and claudin-5 expression were measured by Western blot. The blot is representative of three blots from three independent experiments. *p<0.05 vs control. #p<0.05 vs LPS alone.
|
AMPK activation by resveratrol inhibited LPS-induced oxidative stress in HBMECs
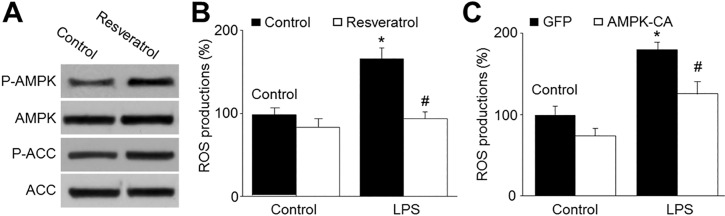 | Fig. 4AMPK activation by resveratrol reduces LPS-enhanced production of reactive oxygen species in HBMECs.(A) Cultured HBMECs were treated with resveratrol (10 µM) for 24 hours. The levels of pAMPK and pACC were measured by Western blotting. The picture is a representative picture from 3 independent experiments. (B) Cultured HBMECs were pretreated with resveratrol (10 µM) for 30 min and then co-incubated with LPS (1 µg/ml) for 24 hours. ROS productions were measured by DHE/HPLC. Data are expressed as the mean±SEM. N is 5 in each group. *p<0.05 vs control. #p<0.05 vs LPS alone. (C) Cultured HBMECs were infected with adenovirus containing constitutively active AMPK (AMPK-CA) mutant for 48 hours and then incubated with LPS (1 µg/ml) for 24 hours. ROS productions were measured by DHE/HPLC. Data are expressed as the mean±SEM. N is 5 in each group. *p<0.05 vs GFP alone. #p<0.05 vs LPS alone.
|
Resveratrol via AMPK activation prevented LPS-induced reduction in tight-junction proteins
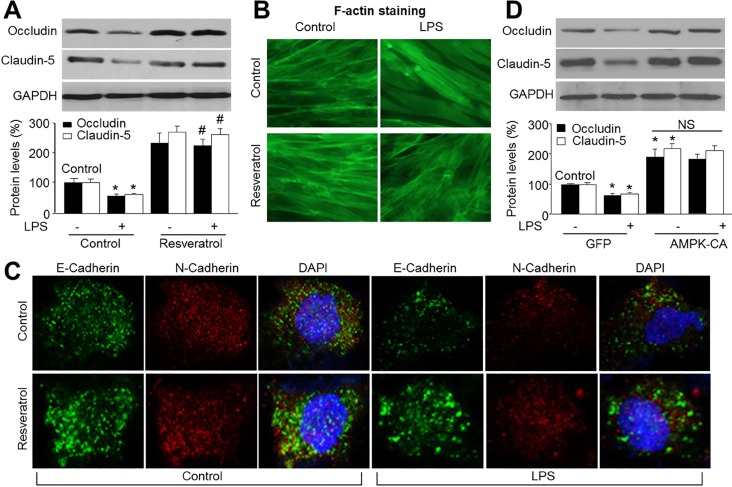 | Fig. 5AMPK activation by resveratrol prevents LPS-induced loss of tight-junction proteins.(A~C) Cultured HBMECs were pretreated with resveratrol (10 µM) for 30 min and then co-incubated with LPS (1 µg/ml) for 24 hours. (A) Expressions of tight junction protein, occludin and claudin-5, were measured by Western blot. Data are expressed as the mean±SEM. N is 3 in each group. *p<0.05 vs control. #p<0.05 vs LPS alone. NS indicates no significance. (B)The morphology of tight-junction was determined by using staining F-actin. (C) Double-labeled immunofluorescence analysis of E-Cadherin (green) and N-Cadherin (red) proteins. Blue, DAPI stained nucleus. (D) Cultured HBMECs were infected with adenovirus containing constitutively active AMPK (AMPK-CA) mutant for 48 hours and then incubated with LPS (1 µg/ml) for 24 hours. Expressions of tight junction protein, occludin and claudin-5, were measured by Western blot. Data are expressed as the mean±SEM. N is 3 in each group. *p<0.05 vs GFP alone. NS indicates no significance.
|
AMPK activation prevented LPS-induced upregulation of NAD(P)H oxidase
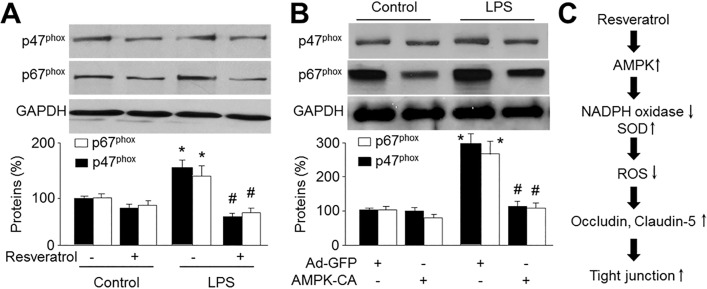 | Fig. 6AMPK activation prevents LPS-induced upregulation of NAD(P)H oxidase subunits.(A) Cultured HBMECs were pretreated with resveratrol (10 µM) for 30 min and then co-incubated with LPS (1 µg/ml) for 24 hours. Expressions of NAD(P)H oxidase subunits, p47phox and p67phox, were measured by Western blot. Data are expressed as the mean±SEM. N is 3 in each group. *p<0.05 vs control. #p<0.05 vs LPS alone. NS indicates no significance. (B) Cultured HBMECs were infected with adenovirus containing constitutively active AMPK (AMPK-CA) mutant for 48 hours and then incubated with LPS (1 µg/ml) for 24 hours. Expressions of NAD(P)H oxidase subunits, p47phox and p67phox, were measured by Western blot. Data are expressed as the mean±SEM. N is 3 in each group. *p<0.05 vs GFP alone. NS indicates no significance. (C) Proposed molecular mechanism for protective effect of AMPK activation on BBB integrity.
|



