Abstract
Regulators of G-protein signaling (RGS) proteins are regulators of Ca2+ signaling that accelerate the GTPase activity of the G-protein α-subunit. RGS1, RGS2, RGS4, and RGS16 are expressed in the pancreas, and RGS2 regulates G-protein coupled receptor (GPCR)-induced Ca2+ oscillations. However, the role of RGS4 in Ca2+ signaling in pancreatic acinar cells is unknown. In this study, we investigated the mechanism of GPCR-induced Ca2+ signaling in pancreatic acinar cells derived from RGS4-/- mice. RGS4-/- acinar cells showed an enhanced stimulus intensity response to a muscarinic receptor agonist in pancreatic acinar cells. Moreover, deletion of RGS4 increased the frequency of Ca2+ oscillations. RGS4-/- cells also showed increased expression of sarco/endoplasmic reticulum Ca2+ ATPase type 2. However, there were no significant alterations, such as Ca2+ signaling in treated high dose of agonist and its related amylase secretion activity, in acinar cells from RGS4-/- mice. These results indicate that RGS4 protein regulates Ca2+ signaling in mouse pancreatic acinar cells.
Following the stimulation of G-protein coupled receptors (GPCRs) or receptor tyrosine kinases (RTKs) by hormones, neurotransmitters, stretch, or cytokines [1] activated Gα subunits stimulate phospholipase C β (PLCβ), and phosphorylated RTKs stimulate PLCγ, respectively. Inositol 1,4,5-triphosphate (IP3) generated by PLC [2-4] evokes Ca2+ release from the endoplasmic reticulum (ER), and depletion of Ca2+ from the ER activates the entry of store-operated Ca2+[1]. In this way, the cytosolic concentration of intracellular Ca2+ is increased, which plays various roles in fertilization, proliferation, development, learning and memory, contraction and secretion, as well as initiation of apoptosis accompanied by cytotoxic effects [1,4,5]. Finally, increased levels of Ca2+ lead to activation of the sarco/ER Ca2+ ATPase (SERCA), and the plasma membrane Ca2+ ATPase (PMCA) pump removes Ca2+ from the cytosol [1,4].
Ca2+ oscillation is an important phenomenon in Ca2+ signaling because a weak physiological stimulus generates transient repetitive oscillations in intracellular Ca2+ concentration ([Ca2+]i) [6]. There are two theories to explain Ca2+ oscillation. One theory is based on an IP3 receptor-related adaptation model. In this model, IP3 affinity to IP3 receptors is related to [Ca2+]i. Therefore, the generation of Ca2+ oscillations is explained biophysically [7]. The other theory is a biochemical model, which is explained by oscillations of intracellular amounts of IP3; this phenomenon itself leads to Ca2+ oscillations [8]. In addition, regulators of G-protein signaling (RGS) proteins are regarded as components of this biochemical mechanism [9].
Several types of RGS proteins exist; the homolog of RGS4 protein, SST2, was discovered first in Saccharomyces cerevisiae [10,11]. Homologs of SST2 were found in various species, including Caenorhabditis elegans [12] and Homo sapiens [13]. RGS proteins were named because of their biochemical function of activating GTPases [14]. RGS proteins inhibit GPCRs via their GTPase activity which accelerates GTP hydrolysis [14]. In mammals, there are many members of the RGS protein family, and some types of RGS proteins have tissue specificity [9].
Pancreatic acinar cells are a good model in which to study Ca2+ signaling because cholecystokinin (CCK) and acetylcholine induce Ca2+ oscillations that are initiated in the pancreatic secretory granules; these cells have characteristics that are regulated in both a spatial and temporal manner [15,16]. Exocytosis of secretory granules containing digestive enzymes occurs via Ca2+ oscillations [15-17]. It is currently thought that RGS1, RGS2, RGS4, and RGS16 are expressed in pancreatic acinar cells [18]. Increased steady-state levels of IP3 were shown to lead to an increased frequency of [Ca2+]i oscillations in RGS2 knock-out mice [19]. Nevertheless, the roles of other RGS proteins in pancreatic acinar cells have not been investigated. The GTPase accelerating activity of RGS4 can be stimulated by Homer 2, which preferentially binds PLCβ in pancreatic acinar extracts [20]. In pancreatic islets, RGS4 deficiency had an effect on insulin release caused by the activation of other β-cell GPCRs. Additionally, treatment of mutant mice selectively lacking RGS4 in pancreatic β-cells treated with a muscarinic agonist (i.e., bethanechol) led to increased plasma insulin and reduced blood glucose levels [21]. From these results, we inferred that RGS4 protein might have an important role in pancreatic acinar cells.
To reveal the function of RGS4 protein in pancreatic acinar cells, we investigated the mechanism of GPCR-induced Ca2+ signaling in pancreatic acinar cells derived from RGS4-/- mice. We found that mutant mice demonstrated more frequent Ca2+ signaling oscillations, more robust Ca2+ mobilization, and increased SERCA2b expression. Our findings suggest that RGS4 protein can regulate Ca2+ signaling in pancreatic acinar cells.
Fura-2/AM was purchased from Teflabs (Austin, TX, USA); CCK octapeptides (sulfated) were purchased from Tocris Biosciences (Bristol, BS11 0QL, UK). Collagenase P was purchased from Roche (Indianapolis, IN, USA). All other chemicals were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Anti-PMCA (5F10) antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Anti-IP3R2 and anti-SERCA2b antibodies were from AbFrontier (Seoul, Korea). Anti-IP3R3 antibodies were from BD Transduction Laboratories (San Jose, CA, USA). Anti-β-actin was from Sigma-Aldrich.
Wild-type (WT) and RGS4 mutant (RGS4-/-) mice with a C57BL/6 background were purchased from Jackson Laboratories, All experiments were performed on adult male C57BL/6 or RGS4 knock-out mice (2 to 6 months of age) that were maintained on a 12-h day/night cycle with normal mouse chow and water provided ad libitum. All procedures involving animals were performed according to the guidelines of the Yonsei University College of Dentistry, Intramural Animal Use and Care Committee. Mice were sacrificed by cervical dislocation under CO2 anesthesia. The cells were prepared from the pancreases of WT and RGS4-/- mice by limited collagenase digestion as previously described [22]. Following isolation, the acinar cells were suspended in an extracellular physiologic salt solution (PSS), the composition of which was as follows (in mM): 140 NaCl, 5 KCl, 1 MgCl2, 1 CaCl2, 10 HEPES, and 10 glucose, adjusted to pH 7.4 with 10 N NaOH and to 310 mOsm with 5 M NaCl.
Pancreatic acinar cells from WT and RGS4-/- mice were loaded with 4μM fura-2/AM and 0.05% pluronic acid F-127 for 30 min in PSS at room temperature. Fura-2/AM fluorescence was measured at an excitation wavelength of 340/380 nm, and emission was measured at 510 nm (ratio=F340/F380) using an imaging system (Molecular Devices, CA, USA). The emitted fluorescence was monitored using a CCD camera (CoolSNAP HQ, AZ, USA) attached to an inverted microscope. Fluorescence images were obtained at 2-s intervals. All data were analyzed using the MetaFluor software (Molecular Devices, Downingtown, PA, USA).
Protein extracts were prepared from isolated pancreatic acini from WT and RGS4-/- mice as follows. Pure acinar cells were lysed in a buffer containing (in mM): 150 NaCl, 10 Tris (pH 7.8 with HCl), 1 EDTA, 1% NP-40, 0.1% SDS, and a protease inhibitor mixture (2 Na3VO4, 10 NaF, 10μg/ml leupeptin, and 10μg/ml phenylmethylsulfonyl fluoride). The samples were probed overnight with 1:1,000 dilutions of antibodies against PMCA (5F10), IP3R2, IP3R3, SERCA2b, and β-actin at 4℃ and then separated by SDS-PAGE.
Animals were allowed water but starved for 24 h prior to the experiment. Each acinar cell was stimulated with equal concentrations of carbachol used in the [Ca2+]i measurement study. Acinar cells were incubated with carbachol for 20 min in a shaking incubator at 37℃ and 60 rpm. Acinar cells were lysed by sonication. The lysates were clarified by centrifugation at 13,000 rpm for 10 min. The total amylase content or content of amylase released into the medium was determined by the method described by Bernfeld [23]. Aliquots of the incubation medium and the supernatants of the homogenized cells were incubated with a 0.5% starch suspension for 10 min at 37℃. Absorbance was measured at 540 nm. Amylase activity in the medium was expressed as a percentage of the total activity.
Total RNA was extracted from isolated pancreatic acinar cells using Trizol reagent (Sigma-Aldrich) according to the manufacturer's instructions. RT-PCR was performed using a SuperScript III RT kit (Sigma-Aldrich) and oligo-dTs (Fermentas, MA, USA). For PCR analysis of SERCA2b, the following primers were used: forward: 5'-TCGGAATACAGCGAGGAGAACATT-3'; reverse: 5'-TCCCTGCCTCTGTGTGAGAATTAG-3'. PCR was initiated by a 5-min incubation of the samples at 94℃, preceded by 25 cycles of 30 s at 94℃, 30 s at 58℃ for annealing, and 30 s at 72℃. After 25 cycles, samples were incubated for 10 min at 72℃ for complete extension. No reverse transcription control was performed using the same protocol, except for a control with no reverse transcriptase added.
To investigate the role of RGS4, we treated acinar cells with different concentrations of carbachol, an agonist of muscarinic receptors. After the initial period of carbachol treatment, its concentration was immediately increased to the maximum level. As shown in Fig. 1, the amount of mobilized Ca2+ ions in the time between the two different concentrations (i.e., initial and maximum) of agonist was measured. Consequently, significant differences in Ca2+ concentrations were observed after treatment with 0.1μM carbachol (WT, 10.3±3.17; RGS4-/-, 21.95±1.63, n=5, p< 0.05) and with 3μM carbachol (WT: 79.46±3.27; RGS4-/-: 94.74±1.03, n=5, p<0.01). No differences in Ca2+ concentrations were observed at levels of 100μM carbachol or higher.
Deletion of RGS4 disturbs normal Ca2+ oscillation patterns due to the reduction in its GTPase accelerating activity. We tested 1μM carbachol and 10 pM CCK in pancreatic acinar cells because these are the known physiologic concentrations of these agonists. As shown in Fig. 2A, the frequency of Ca2+ oscillations was observed to be 0.181±0.020 (spikes/min) in WT and 0.294±0.024 (spikes/min, n=16, p<0.01) in RGS4-/-acinar cells. In the same protocol, we observed the frequency of Ca2+ oscillations in response to 1μM carbachol. Ca2+ oscillations were more frequent in RGS4-/- knock-out mice than in WT mice [WT: 0.830±0.131 (spikes/min); RGS4-/-: 1.072±0.099 (spikes/min)].
It is possible that the increases in Ca2+ oscillation frequency and Ca2+ mobilization are due to the different protein expression levels of SERCA2b related to the Ca2+ oscillation pathways. To confirm this hypothesis, we isolated RGS4-/- pancreatic acini and performed western blotting analysis. We observed that the expression levels of PMCA, IP3R2, and IP3R3 were the same between WT and RGS4-/- cells; however, there was an increase in SERCA2b gene expression in RGS4-/- cells (Fig. 3A). Using RT-PCR for a detailed comparison of mRNA expression levels between WT and RGS4-/- cells, we observed an increased SERCA2b gene expression in RGS4-/- pancreatic acini (Fig. 3B) (n=4, p<0.05).
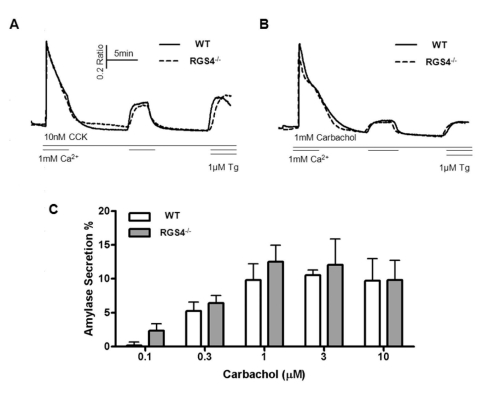
As suggested in Figs. 1 and 2, increases in Ca2+ mobilization and Ca2+ oscillation frequency can exert an effect of Ca2+ influx and efflux in pancreatic acini. We removed Ca2+ ions from the external solution after initial treatment with agonist and re-introduced the Ca2+ ions during treatment with maximum levels of agonist (1μM carbachol or 10 nM CCK) (Fig. 4A, B). WT and RGS4-/- cells did not show any differences in transient cytosolic Ca2+ levels. Exocytosis and fluid secretion occurs during Ca2+ signaling in stimulated secretory cells. Therefore, we tested the levels of secreted amylase in WT and RGS4-/- acinar cells. Levels of secreted amylase were slightly increased in RGS4-/- cells compared with WT cells; however, the differences were not statistically significant (Fig. 4C).
In this study, we demonstrated different Ca2+ signaling patterns in WT and RGS4-/- pancreatic acinar cells. First, Ca2+ mobilization was more prominent in pancreatic acinar cells of RGS4-/- mice compared with WT. This effect could be the result of reduced GTPase accelerating activity in RGS4-/- cells, which is the normal physiological function of the RGS4 protein. The effect could also be related to increased SERCA2b expression levels in RGS4-/- cells. Both factors could influence Ca2+ mobilization; however, based on our current experiments, we cannot conclude which is the major factor.
Nevertheless, this study indicates that RGS4-/- pancreatic acinar cells are more sensitive to carbachol-induced Ca2+ signaling. Therefore, we hypothesized that the frequency of Ca2+ oscillations would increase in these cells. Results of the calcium mobilization test can be explained in the same manner. Ca2+ oscillation is regarded as a key component in signal transmission and is related to the secretory process in pancreatic acinar cells [15,16]. Therefore, there would be an increase in the secretory function of cells that show an increase in the frequency of Ca2+ oscillations.
To confirm this hypothesis, we performed amylase secretion assays because pancreatic acinar cells secrete α-amylase during cytosolic Ca2+ oscillations. Unfortunately, we did not find any differences in amylase secretion between WT and RGS4-/- cells. This result indicates that the increase in Ca2+ oscillation frequency is insufficient to change the secretory function of the acinar cells.
The next goal of this study was to evaluate the expression levels of molecules such as IP3R2, IP3R3, PMCA, and SERCA2b in WT and RGS4-/- acinar cells. A change in the frequency of Ca2+ oscillations was observed due to altered expression levels of these molecules. According to western blotting analysis, IP3R2, IP3R3, and PMCA exprxession levels were similar, but SERCA2b expression was slightly increased in RGS4-/- acinar cells. Quantitative PCR further confirmed these results, and these results suggest that deletion of RGS4 is probably related to SERCA2b gene expression in pancreatic acini cell. From these results, we inferred that deletion of RGS4 affects SERCA2b expression through an uncertain mechanism. We speculate that this phenomenon is an example of adaptation because cytosolic Ca2+ levels in RGS4-/- cells are higher and last longer than those in WT cells. In this situation, RGS4-/- cells need to clear Ca2+ ions from the cytosol more quickly.
The present study adds new insight into the signal transduction pathway of pancreatic acinar cells that regulate secretory function. RGS4 is a member of the B/R4 subfamily of RGS proteins, acting as a GTPase activating protein (GAP) for Gq- and Gi-type G-protein α-subunits [24,25]. In WT pancreatic acini, RGS4 inhibited certain Gq subunits, such as those in muscarinic receptors. For this reason, RGS4-/- pancreatic acini may display enhanced activity of muscarinic receptors. Thus, increased Ca2+ oscillation frequency and mobilization in RGS4-/- acinar cells could be a result of function of RGS4.
And results of the increased SERCA2b expression, released Ca2+ would be quickly uptake to ER, and thereby makes the RGS4-/- acinar cells more ready for next stimulation. This change would be another explanation of increased frequency of Ca2+ oscillation.
In RGS4-/- mouse pancreatic acinar cells, the frequency of Ca2+ oscillations increased along with the induction of SERCA2b expression. In addition, levels of secretory amylase were slightly higher in RGS4-/- cells. Elevated cytosolic Ca2+ levels can induce cellular apoptosis [5], and increased concentrations of cytosolic Ca2+ lead to increased concentrations of Ca2+ in the mitochondrial matrix, thereby inducing apoptosis [26]. For these reasons, viable cells must clear cytosolic Ca2+ rapidly. In RGS4-/- pancreatic acini, more robust Ca2+ release could be induced by physiologic stimuli when compared with WT cells. Hence, we suggest that the potential role of RGS4 in acinar cells is to regulate SERCA2b expression levels and the frequency of Ca2+ oscillations.
Increased activity of store-operated Ca2+ channels (SOCs) induces Ca2+ oscillations [27]. In this context, increased frequency of Ca2+ oscillations could be unrelated with SERCA2b expression. Nevertheless, in the present study, patterns of Ca2+ influx/efflux were similar between WT and RGS4-/- acinar cells after treatment with GPCR agonists. This result indicates that SOCs are not influenced by Ca2+ signaling and that increases in the frequency of Ca2+ oscillations would be due to increased expression of SERCA2b.
All alterations induced by RGS4 deletion in pancreatic acini could be a result of the modified physiologic function of pancreatic acinar cells. To confirm this hypothesis, we performed an amylase secretion assay. The average secretory value was slightly higher in RGS4-/- acinar cells than in WT cells, although the difference was not significant, indicating that the alteration in Ca2+ signaling induced by the deletion of RGS4 is insufficient to increase amylase secretion.
Our study provides insights into the function of RGS4 in pancreatic acinar cells. We believe that these data will help in the understanding of the function of Ca2+ dynamics and the physiology of the pancreas.
ACKNOWLEDGEMENTS
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2011-0001167, 2011-0025684).
References
1. Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol. 2003; 4:517–529. PMID: 12838335.

2. Marshall CJ. Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell. 1995; 80:179–185. PMID: 7834738.

3. Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol. 2000; 1:11–21. PMID: 11413485.

4. Kiselyov K, Shin DM, Muallem S. Signalling specificity in GPCR-dependent Ca2+ signalling. Cell Signal. 2003; 15:243–253. PMID: 12531423.
5. Mattson MP, Chan SL. Calcium orchestrates apoptosis. Nat Cell Biol. 2003; 5:1041–1043. PMID: 14647298.

6. Thorn P, Lawrie AM, Smith PM, Gallacher DV, Petersen OH. Ca2+ oscillations in pancreatic acinar cells: spatiotemporal relationships and functional implications. Cell Calcium. 1993; 14:746–757. PMID: 8131191.
7. Bezprozvanny I, Watras J, Ehrlich BE. Bell-shaped calcium-response curves of Ins(1,4,5)P3- and calcium-gated channels from endoplasmic reticulum of cerebellum. Nature. 1991; 351:751–754. PMID: 1648178.
8. Wakui M, Potter BV, Petersen OH. Pulsatile intracellular calcium release does not depend on fluctuations in inositol trisphosphate concentration. Nature. 1989; 339:317–320. PMID: 2498663.

9. Willars GB. Mammalian RGS proteins: multifunctional regulators of cellular signalling. Semin Cell Dev Biol. 2006; 17:363–376. PMID: 16687250.

10. Chan RK, Otte CA. Physiological characterization of Saccharomyces cerevisiae mutants supersensitive to G1 arrest by a factor and alpha factor pheromones. Mol Cell Biol. 1982; 2:21–29. PMID: 7050666.

11. Chan RK, Otte CA. Isolation and genetic analysis of Saccharomyces cerevisiae mutants supersensitive to G1 arrest by a factor and alpha factor pheromones. Mol Cell Biol. 1982; 2:11–20. PMID: 7050665.

12. Koelle MR, Horvitz HR. EGL-10 regulates G protein signaling in the C. elegans nervous system and shares a conserved domain with many mammalian proteins. Cell. 1996; 84:115–125. PMID: 8548815.

13. De Vries L, Mousli M, Wurmser A, Farquhar MG. GAIP, a protein that specifically interacts with the trimeric G protein G alpha i3, is a member of a protein family with a highly conserved core domain. Proc Natl Acad Sci USA. 1995; 92:11916–11920. PMID: 8524874.

14. Berman DM, Wilkie TM, Gilman AG. GAIP and RGS4 are GTPase-activating proteins for the Gi subfamily of G protein alpha subunits. Cell. 1996; 86:445–452. PMID: 8756726.
15. Kasai H, Li YX, Miyashita Y. Subcellular distribution of Ca2+ release channels underlying Ca2+ waves and oscillations in exocrine pancreas. Cell. 1993; 74:669–677. PMID: 8395348.
16. Thorn P, Lawrie AM, Smith PM, Gallacher DV, Petersen OH. Local and global cytosolic Ca2+ oscillations in exocrine cells evoked by agonists and inositol trisphosphate. Cell. 1993; 74:661–668. PMID: 8395347.
17. Choi KJ, Cho DS, Kim JY, Kim BJ, Lee KM, Kim SH, Kim DK, Kim SH, Park HS. Ca2+-induced Ca2+ release from internal stores in INS-1 rat insulinoma cells. Korean J Physiol Pharmacol. 2011; 15:53–59. PMID: 21461241.
18. Luo X, Ahn W, Muallem S, Zeng W. Analyses of RGS protein control of agonist-evoked Ca2+ signaling. Methods Enzymol. 2004; 389:119–130. PMID: 15313563.
19. Wang X, Huang G, Luo X, Penninger JM, Muallem S. Role of regulator of G protein signaling 2 (RGS2) in Ca2+ oscillations and adaptation of Ca2+ signaling to reduce excitability of RGS2-/- cells. J Biol Chem. 2004; 279:41642–41649. PMID: 15292238.
20. Shin DM, Dehoff M, Luo X, Kang SH, Tu J, Nayak SK, Ross EM, Worley PF, Muallem S. Homer 2 tunes G protein-coupled receptors stimulus intensity by regulating RGS proteins and PLCbeta GAP activities. J Cell Biol. 2003; 162:293–303. PMID: 12860966.
21. Ruiz de Azua I, Scarselli M, Rosemond E, Gautam D, Jou W, Gavrilova O, Ebert PJ, Levitt P, Wess J. RGS4 is a negative regulator of insulin release from pancreatic beta-cells in vitro and in vivo. Proc Natl Acad Sci USA. 2010; 107:7999–8004. PMID: 20385802.
22. Zeng W, Lee MG, Yan M, Diaz J, Benjamin I, Marino CR, Kopito R, Freedman S, Cotton C, Muallem S, Thomas P. Immuno and functional characterization of CFTR in submandibular and pancreatic acinar and duct cells. Am J Physiol. 1997; 273:C442–C455. PMID: 9277342.

23. Bernfeld P. Enzymes of starch degradation and synthesis. Adv Enzymol Relat Subj Biochem. 1951; 12:379–428. PMID: 14885023.

24. Ross EM, Wilkie TM. GTPase-activating proteins for heterotrimeric G proteins: regulators of G protein signaling (RGS) and RGS-like proteins. Annu Rev Biochem. 2000; 69:795–827. PMID: 10966476.

25. Hollinger S, Hepler JR. Cellular regulation of RGS proteins: modulators and integrators of G protein signaling. Pharmacol Rev. 2002; 54:527–559. PMID: 12223533.

26. Giacomello M, Drago I, Pizzo P, Pozzan T. Mitochondrial Ca2+ as a key regulator of cell life and death. Cell Death Differ. 2007; 14:1267–1274. PMID: 17431419.
27. Mancarella S, Wang Y, Gill DL. Calcium signals: STIM dynamics mediate spatially unique oscillations. Curr Biol. 2009; 19:R950–R952. PMID: 19889372.

Fig. 1
Measurement of Ca2+ mobilization in wild-type and RGS4-/- pancreatic acinar cells. Briefly, pancreatic acinar cells were isolated from WT and RGS4-/- mice and treated with various concentrations of carbachol (i.e., 0.05 to 100µM). The treatment concentration was then immediately changed to the maximum concentration (i.e., 1 mM). Ca2+ mobilization was calculated using the ratio of the calculated area below the graph of the initial carbachol concentration and that of the maximum carbachol concentration (i.e., 1 mM). Intracellular Ca2+ concentrations were measured by fluorescence microscopy using fura2-AM. In RGS4-/- cells (B), a more sensitive and enhanced response was observed than in WT cells (A). Graphical representation of the data is presented in (C). Car, carbachol.
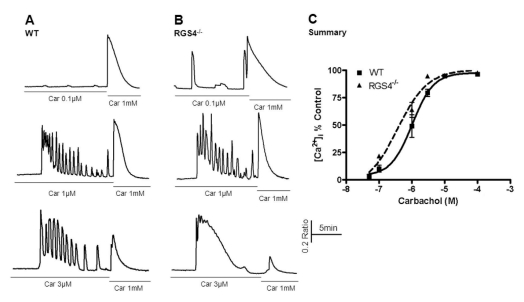
Fig. 2
Measurement of Ca2+ oscillations in WT and RGS4-/- cells. Effects on Ca2+ oscillations after treatment of pancreatic acinar cells with 10 pM cholecystokinin (CCK) (A); graphically represented in (B). Ca2+ oscillation frequency increased in RGS4-/- cells (p<0.01). Ca2+ oscillation frequency in 0.5µM carbachol-treated cells (C); graphically represented in (D).
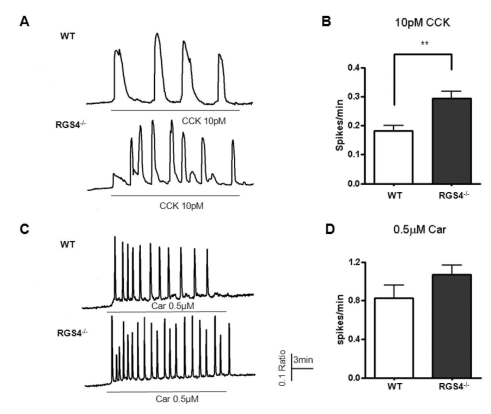
Fig. 3
Expression of Ca2+ signaling molecules in WT and RGS4-/- cells. Western blotting was performed using anti-SERCA2b, anti-PMCA (5F10), anti-IP3R2, and anti-IP3R3 antibodies in isolated pancreatic acinar cells (A). Increases in SERCA2b mRNA (n=4, p<0.05) were confirmed by RT-PCR (B).
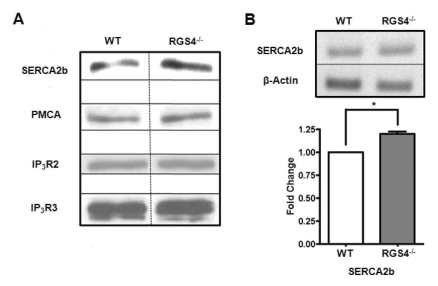
Fig. 4
Measurement of Ca2+ influx/efflux and amylase secretion. During the treatment with high doses of CCK (A) and carbachol (B), regulation of cytosol Ca2+ was observed. There were no significant differences between WT and RGS4-/- acinar cells. Percent average amylase secretion was slightly higher in RGS4-/- cells than in WT cells (n=4); however, the differences were not statistically significant (C).




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download