Abstract
ACKNOWLEDGMENTS
References
Table 1
Agreement of respondents (a total of 99 primary physician) for the survey “Do you agree that existing guidelines are insufficient to answer the following question, and a new guideline to reflect real-practice is needed”
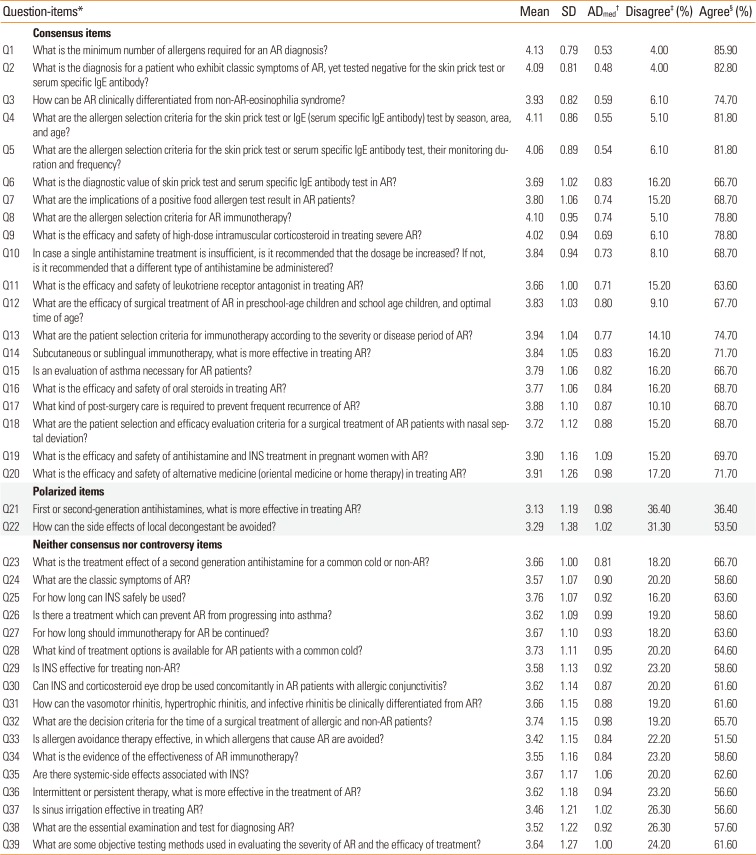
*Criteria of question-items: “Consensus items” if less than 25% of responders indicated neutral opinion and if the percentage of agreement was at least 4 times as large as the percentage of disagreement; “Polarized items” if over 30% of the responders indicated agreement and if over 30% of responders indicated disagreement; “Neither consensus nor controversy” if it was not included in “Consensus items” or “Polarized items.”; †ADmed values less than or equal to 0.833 were interpreted as indicating acceptable interrater agreement; ‡Disagree: disagreement was considered present if the responders choosing 2 (disagree) or 1 (strongly disagree) for each statement; §Agree: agreement was considered present if the responders choosing 4 (agree) or 5 (strongly agree) for each statement. Overall degree of agreement was ascertained using a 5-point Likert scale with 1=strongly disagree and 5=strongly agree.
SD, standard deviation; AR, allergic rhinitis; INS, intranasal corticosteroid.
Table 2
Specialty-agreement gaps for the question “Do you agree that existing guidelines is insufficient to answer the following question, and new guideline to reflect real-practice is needed”

*Criteria of question-items: “Consensus items” if less than 25% of responders indicated neutral opinion and if the percentage of agreement was at least 4 times as large as the percentage of disagreement; “Polarized items” if over 30% of the responders indicated agreement and if over 30% of responders indicated disagreement; “Neither consensus nor controversy” if it was not included in “Consensus items” or “Polarized items.”; †Agree: agreement was considered present if the responders choosing 4 (agree) or 5 (strongly agree) for each statement. The overall degree of agreement was ascertained using a 5-point Likert scale with 1=strongly disagree and 5=strongly agree; ‡ADmed values less than or equal to 0.833 were interpreted as indicating acceptable interrater agreement.
AR, allergic rhinitis; PD, pediatrics; ORL, otorhinolaryngology; IM, internal medicine; INS, intranasal corticosteroid.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download