Abstract
Interleukin-33 (IL-33) is the 11th member of IL-1 cytokine family which includes IL-1 and IL-18. Unlike IL-1β and IL-18, IL-33 is suggested to function as an alarmin that is released upon endothelial or epithelial cell damage and may not enhance acquired immune responses through activation of inflammasome. ST2, a IL-33 receptor component, is preferentially expressed by T-helper type (Th) 2 cells, mast cells, eosinophils and basophils, compared to Th1 cells, Th17 cells and neutrophils. Thus, IL-33 profoundly enhances allergic inflammation through increased expression of proallergic cytokines and chemokines. Indeed, IL-33 and its receptor genes are recognized as the most susceptible genes for asthma by several recent genomewide association studies. It has also recently been shown that IL-33 plays a crucial role in innate eosinophilic airway inflammation rather than acquired immune responses such as IgE production. As such, IL-33 provides a unique therapeutic way for asthma, i.e., ameliorating innate airway inflammation.
Interleukin-33 (IL-33), a member of the IL-1 cytokine family, is considered to be crucial for the induction of T-helper type (Th) 2 cell-dominant immune responses such as host defense against nematodes and allergic diseases.1 IL-33 was originally identified as "DVS27", a gene upregulated in vasospastic cerebral arteries after subarachnoid hemorrhage2 and as a nuclear factor, "nuclear factor from high endothelial venules (NF-HEV)", which is expressed in endothelial cell nuclei.3
IL-33 receptor was first identified as an IL-1 receptor-like molecule and termed as ST2 (the gene symbol was termed as IL1RL1) by Tominaga in 1989.4 ST2 was subsequently found to be preferentially expressed in Th2 cells and started to attract many researchers involved in allergy.5 In 2005, DVS27 was rediscovered as the 11th member in the IL-1 family of cytokines, which includes IL-1α, IL-1β, and IL-18, by computationally searching for the sequences containing β-trefoil structure seen in IL-1- and FGF-like proteins, and termed as IL1F11 or IL-33.6
As the receptors for the other IL-1-related cytokines, the IL-33 receptor is formed heterodimeric molecules consisting of ST2 and IL-1 receptor accessory protein (IL-1RAcP; Fig. 1). IL-1RAcP is also known as a common component of receptors for IL-1α, IL-1β, IL-1F6, IL-1F8, and IL-1F9.1
The two major products of ST2 genes (IL1RL1), i.e., transmembrane form ST2 (ST2 or ST2L) and soluble form ST2 (sST2) are produced by alternative splicing under the control of two distinct promoters. ST2 is considered to be the functional component for induction of IL-33 bioactivities, while sST2 act as a decoy receptor for IL-33 like soluble IL-1Rs for IL-1.1
The signal transduction downstream of IL-33 receptor is mediated by common adapter molecules to that of the other IL-1 receptor family such as IL-1R and IL-18R. The binding of IL-33 to IL-33 receptor results in the recruitment of MyD88 to the Toll-interleukin-1 receptor domain in cytoplasmic region of ST2, leading to the induction of inflammatory mediators by activating transcription factors such as NF-κB and AP-1 through IRAK, TRAF6 and/or MAP kinases, like other IL-1 family receptor or Toll-like receptor (TLR) activation.6
In contrast to the other IL-1 family cytokines except IL-1α, IL-33 is localized to the nucleus of human epithelial and endothelial cells2 and mouse bone-marrow derived cultured mast cells7 by binding to chromatin via a homeodomain (helix-turn-helix-like motif) and nuclear localization signal in amino-terminus.8 Although the pathophysiological role of IL-33 as a nuclear factor is not fully understood, IL-33 binds to the acidic pocket of dimeric histone H2A-H2B at the surface of nucleosomes, resulting in the suppression of the gene transcription at least in the in vitro reporter assay system.8
During host defense against pathogens, innate-type immune cells recognize pathogen-associated molecular patterns via TLRs, resulting in induction of inflammation. In addition, endogenous proinflammatory factors called "damage associated molecular patterns (DAMPs)" (also called "alarmin"), which are released by necrotic cells in tissue injury during trauma and/or infection, also provoke local and/or systemic inflammation by alerting acquired-type immune cells as an endogenous danger signal.9 For example, high-mobility group box 1 (HMGB1), which is originally identified as a nuclear factor as a transcriptional regulator, is released by macrophages in response to lipopolysaccharide, leading to the induction of inflammation.10 Like HMGB1, recent several lines of evidence suggest that IL-33, which also localizes in nucleus, act as a DAMP/alarmin.11
IL-33 was originally considered to be secreted by the activation of NACHT, LRR and PYD containing protein (NLRP)-mediated inflammasomes like IL-1β and IL-18 since it is cleaved from pro-IL-33 by caspase-1 in vitro.6 However, pro-IL-33 does not have a typical cleavage site seen in pro-IL-1β and -IL-18, and caspase-1 was found to proteolytically cleave pro-IL-33 at the cytokine motif, but not the intermediate region between helix-turn-helix motif and cytokine motif, resulting in the inactivation of IL-33.11-13 Like caspase-1, both caspase-3 and caspase-7 have an ability to cleave pro-IL-33 during apoptosis, in which apoptotic cells do not induce inflammation generally, and the processed IL-33 by these caspases do not have biological activities.11,12
On the other hand, biologically active pro-IL-33 can be released by necrotic cells without any processes by caspase-1, -3, -7, -8, and calpain.7,11-13 For example, pro-IL-33 can induce mouse mast cell activation to produce cytokines.13 These observations suggest that pro-IL-33 released by necrotic cells during tissue injury may have a potential role in induction of inflammation as a DAMP/alarmin.
Asthma is an inflammatory disease characterized by infiltration of the airway wall with a variety of immune cells and inflammatory cells such as Th2 cells, mast cells and eosinophils. However, a key component of asthma is the structural change that involves all of the elements of the airway wall associated with activation of the epithelial-mesenchymal trophic unit.14
Activated epithelial and mesenchymal cells generate a range of growth factors associated with airway remodeling and cytokines manipulating the immune response. Although IL-33 is present in the nuclei of various cell types, epithelial cells15 and endothelial cells16 are recognized as the major sources of the cytokine especially when considering the event of tissue damage.
Thus, like thymic stromal lymphopoietin (TSLP),17 IL-33 is now recognized as an epithelial-mesenchymal-derived cytokine manipulating inflammatory and/or immune responses.
It is well established that IL-4 is a key cytokine for the differentiation of Th2 cells from naïve CD4+ T cells. ST2 is predominantly expressed on Th2 cells but not naïve T cells, Th1 cells, Th17 cells and regulatory T cells.18-20 On the other hand, ST2 is not essential for Th2 cell differentiation as shown in the study using ST2-deficient mice; ST2-deficient mice showed the normal development of Th2 cells.21,22 In support for the notion, although IL-33 cannot induce the differentiation of Th2 cells from naïve CD4+ T cells in vitro,23,24 IL-33 can enhance IL-5 and IL-13 production by in vitro-skewed Th2 cells which highly express ST2.6,25,26 Also, Kurowska-Stolarska et al.24 reported that IL-33 induces the differentiation of IL-5+IL-4- CD4+ Th cells from naïve CD4+ T cells independently of IL-4, STAT-6 and GATA-3, which are important factors for the typical Th2 cell differentiation.
Lin- c-Kit+ Sca-1+ natural helper cells dwelling in the gut adipose tissue are a newly identified.29 Natural helper cells constitutively express ST2 and can produce a larger amount of IL-5 and IL-13 rather than basophils and mast cells in response to IL-33. It was shown that IL-33-mediated natural helper cell activation was important for formation of goblet cell hyperplasia during Nippostrongylus brasiliensis infection.29 Similar ST2-expressing non T/non B lymphoid cell types capable of producing IL-5 and IL-13 in response to IL-33, are subsequently identified by other investigators.30,31
Mast cells, which dwell in the mucosal and connective tissues, express c-Kit and high affinity IgE receptors (FcεRI), and induce IgE-mediated immune responses, are also major targets of IL-33. Mouse and human mast cells constitutively express ST2.32-35 Except IL-3 and stem cell factor (SCF, a ligand for c-kit), which are required for mast cell development at least in mouse, IL-33 is the only cytokine among 45 different cytokines which can directly provoke cytokine/chemokine (IL-1β, IL-6, IL-13, TNF, and MCP-1) secretion from mouse bone-marrow derived cultured mast cells without affecting their degranulation.36,37
Like a murine counterpart, IL-33 can induce cytokine and chemokine production, prolong survival and promote cell-adhesion in human cord blood stem cell-derived cultured mast cells.34,35 In addition, IL-33 can augment IgE-mediated cytokine production and degranulation by mouse bone-marrow derived cultured mast cells and human cord blood stem cell-derived cultured mast cells.34-36,38
Another highly FcεRI-expressing cell type, basophils, which circulate in the peripheral blood and are potential primary sources of IL-4,39,40 are also considered as the major target of IL-33. In comparison with Th2 cells and mast cells, human and mouse basophils constitutively express ST2 at the relatively low level on their cell surface.23,26,41,42 On the other hand, the expression of ST2 on the cell surface of basophils is promoted by stimulation with IL-3.26
Like the effect of IL-33 on Th2 cells and mast cells, IL-33 alone can induce the production of cytokine including Th2-type cytokines and chemokines by basophils and promote cell-adhesion and CD11b expression in basophils in human or mice.26,27,41,42 IL-33 does not induce degranulation by basophils directly, while IL-33 synergistically enhances IgE-mediated degranulation by human basophils.26,41
In addition, IL-33 augments immune responses of basophils in human or mice; eotaxin-mediated migration,41 cytokine secretion in the presence of IL-3, which is a growth factor for basophils like mast cells,23,26,27,41-43 and prolongs survival in the presence of IL-3 or GM-CSF.41-43 These observations suggest that IL-33 is a potential activator for basophils by enhancing cytokine and chemokine secretion, recruitment and adhesion.
Peripheral blood eosinophils, compared to neutrophils, are preferentially recruited into the tissue at the site of inflammation in patients with certain IgE-mediated allergic disorders such as asthma. Although ST2 expression was barely detectable on cell surface of peripheral blood eosinophils in human, ST2 mRNA and intracellular ST2 protein were detectable in them.26,44,45 IL-33 can induce the production of superoxide and IL-8 directly, and enhance IL-3, IL-5 or GM-CSF-mediated IL-8 production by human eosinophils.26,44
Like mast cells and basophils, IL-33 enhances adhesion of eosinophils by promoting CD11b expression and survival independently of IL-4, IL-5, and GM-CSF.45 Unlike basophils, IL-33 did not influence eotaxin-mediated migration of eosinophils.45 These observations strongly suggest that IL-33 may contribute to the pathogenesis of certain allergic disorders accompanied by marked accumulation of eosinophils.
IL-33 promotes the development of dendritic cells (DCs) from bone marrow cells.46 It has been shown that DCs derived by the cultivation of murine bone marrow cells in the presence of GM-CSF and IL-4 (that is, bone marrow-derived DCs; BMDCs) express ST2.47 IL-33 enhances the production of IL-6, but not IL-12, by BMDCs and augments the expression of MHC class II and CD86, but not CD80, CD40 and OX40 ligand (OX40L), on the cell surface of BMDCs.47
When naive CD4+ T cells were co-cultured with BMDCs in the presence of IL-33 for 6 to 10 days, IL-5 and IL-13, but not IL-4 and IFN-γ, were detected in the culture supernatant even without TCR engagements. Since the secreting cytokine profiles (IL-5 and IL-13, but not IL-4, production) in the settings (BMDCs + naïve CD4+ T cells + IL-33, no antigens) are similar to those by IL-5-positive IL-4-negative atypical Th2 cell population or innate lymphoid cell types, IL-33 may enhance the induction of these cell types from naïve CD4+ T cells.
Like IL-33, IL-25 and TSLP are known to be epithelial/mesenchymal cytokines inducing Th2-type cytokine-mediated immune responses.48 Contrast to IL-33, TSLP-activated DCs promotes IL-4-producing Th2 cell differentiation from naïve CD4+ T cells in the presence of TCR engagements through OX40L-OX40 interaction at least in part.49,50 IL-25 can enhance TSLP-stimulated DC-mediated Th2 cell expansion.51
Unlike IL-33, both TSLP and IL-25 can induce the differentiation of IL-4-producing Th2 cells from naïve CD4+ T cells after TCR engagements dependently of IL-4-IL-4Rα-STAT6 pathway.52,53 Therefore, these observations suggest that the role of IL-33, TSLP, and IL-25 in T cells and DCs may be different in Th2-type cytokine-mediated immune responses; TSLP and IL-25 may be involved in the preferential induction of antigen-specific IL-4/IL-5/IL-13-producing Th2 cell-mediated immune responses, while IL-33 may contribute to the induction of antigen-non specific Th2 cell-mediated immune responses by inducing IL-5/IL-13-, but not IL-4-, producing atypical Th2 cells or innate lymphoid cells.
Regarding epithelial mesenchymal cell types, Yagami et al.54 have examined IL-33-responsive cells among primary human lung tissue cells. They found that ST2 mRNA was expressed in both endothelial and epithelial cells but not in fibroblasts or smooth muscle cells. Correspondingly, IL-33 promoted IL-8 production by both endothelial and epithelial cells but not by fibroblasts or smooth muscle cells. Transfection of ST2 small interference RNA into both endothelial and epithelial cells significantly reduced the IL-33-dependent upregulation of IL-8, suggesting that IL-33-mediated responses in these cells occur via the ST2 receptor.
While Th2 cytokines, such as IL-4, further enhanced ST2 expression and function in both endothelial and epithelial cells, Th2/eosinophil-related cytokines/chemokines were not produced by these cell types. While the IL-33-mediated production of IL-8 by epithelial cells was almost completely suppressed by corticosteroid treatment, the effect of corticosteroid treatment on the IL-33-mediated responses of endothelial cells was only partial.54
Comprehensive role of IL-33 regarding other cell types and diseases other than asthma has been shown in our previous review article.1
The completion of the Human Genome Project, the HapMap project, and technological advances55 allowed genome-wide association studies (GWAS) to more comprehensively identify the susceptibility genes for asthma. Although asthma is now recognized as a syndrome consisting of heterogeneous disease entities,56 several genes are shown to be susceptible for asthma in GWAS. Recent large-scale GWAS all show the genes for IL-33 (IL33) and ST2 (IL1RL1) are susceptible for asthma onset.57-61 It should be noted that IL33 and IL1RL1 are located in different chromosomes, and that only these two genes are consistently listed as asthma-susceptible genes in these literatures. Interestingly, susceptible genes for atopy (IgE production) were entirely different from those for asthma including IL33 and IL1RL1.58
Thus, IL-33 now attracts much attention from all doctors and investigators who are involved in asthma research.
The levels of soluble ST2 proteins and IL-33 mRNA/proteins are increased in sera and tissues from patients with asthma.27,62-65 Also, intraperitoneal or intranasal administration of IL-33 in mice leads to the induction of inflammation accompanied by eosinophils in mucosa of lung and intestine through the IL-13 and STAT6-dependent pathway.6,23 The levels of soluble ST2 protein and IL-33 mRNA are increased in sera and/or lungs in a murine asthma model of airway inflammation induced by ovalbumin (OVA).66,67
However, the role of ST2 and IL-33 in the induction of OVA-induced airway inflammation in mice is controversial. Especially, apparent discrepancy is often found between the studies using ST2-deficient mice and the studies using mice treated with anti-ST2 and ST2-Fc fusion proteins.1
Respiratory function, eosinophilic airway inflammation and the levels of serum total IgG1 and IgE were normally observed in 129×B6 mixed and BALB/c background-ST2-deficient mice sensitized twice with OVA emulsified with alum.21,24,68 Several investigators reported the effect of anti-ST2 mAb (clone 3E10) on OVA-induced airway inflammation in BALB/c mice (twice sensitization model with OVA/alum). Airway inflammation induced by OVA was attenuated in BALB/c mice treated with the 3E10 anti-ST2 mAb.69,70 Likewise, Th2 responses during OVA-induced airway inflammation were reduced in mice treated with anti-IL-33 polyclonal Ab.71
Adoptive transfer with DO11.10 Th2 cells, which express OVA-specific T cell receptors, into mice results in Th2 cytokine-dependent eosinophilic airway inflammation after intranasal OVA challenge.72 The airway inflammation was exacerbated when BALB/c wild-type mice or BALB/c-Rag-1-deficient mice injected with ST2-deficient DO11.10 Th2 cells were challenged with OVA in comparison with those mice injected with ST2-sufficient DO11.10 Th2 cells.68 It suggests that IL-33 signals on Th2 cells may have a regulatory function in OVA-induced airway inflammation. Contrast with the study using ST2-deficient DO11.10 Th2 cells,68 administration of the 3E10 anti-ST2 mAb or soluble ST2-Fc fusion protein in mice injected with DO11.10 Th2 cells showed the attenuated airway function and inflammation after OVA challenge.19,70
The reason for this discrepancy still remains unclear. However, it will be clarified by examining as to whether the expression of IL1RAcP, which is a signal amplifier and forms receptor complex not only with ST2 but also with IL-1 receptor (IL1R), is excessive in various immune cell types from ST2-deficient mice (Fig. 1).
Since the possibility of excessive IL-1 signaling in ST2 deficient mice cannot be ruled out, generation of IL-33 deficient mice has long been expected. Oboki et al.73 have only recently generated the IL-33 deficient mice. These mice normally develop, suggesting that IL-33 does not play a crucial role as a nuclear factor in physiological development.
During airway inflammation induced by twice OVA with alum sensitization, IL-33-/- mice showed attenuated eosinophil influx into the bronchoalveolar lavage (BAL) fluid and pulmonary inflammation. In contrast, IL-4 and IL-5 levels in the BAL fluid and serum OVA-specific IgE production were only slightly (i.e., not significantly) reduced in IL-33-/- mice after the last OVA challenge. IL-33-deficiency also significantly diminished inflammatory cell influx into the BAL fluid during airway inflammation induced by an extract derived house dust mites (HDM).73
Furthermore, inhalation of papain, a cystein protease allergen having strong homology with HDM allergen, Der 1,74 which can induce airway inflammation even in T/B cell-deficient Rag-2-/- mice, induced strong airway eosinophilia even without sensitization process. Most importantly, papain-induced airway inflammation is profoundly abolished in IL-33-/- mice as well as IL-4-/-IL-13-/- mice. Therefore, the papain-induced innate airway inflammation is IL-33-dependent, and that IL-4 and/or IL-13 derived from innate inflammatory cells but not T cells are important for the event.73
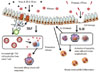
Taken together, IL-33 is important for inducing antigen-dependent Th2-associated local airway inflammation. However, unlike TSLP17 and IL-1, IL-33 is mostly dispensable for antigen-specific Th2 cell differentiation and antigen-specific IgE production (Fig. 2), although the actual asthma pathogenesis cannot be compartmentalized; i.e., IL-33 is capable of stimulating TSLP production.75
Oboki et al.73 also found that IL-33 is involved in the development of dextran-induced colitis accompanied by T cell-independent epithelial cell damage, but not in streptozocin-induced diabetes or Con A-induced hepatitis characterized by T cell-mediated apoptotic tissue destruction. In addition, IL-33 failed to play a substantial role in induction of T cell mediated contact and delayed-type hypersensitivity and autoimmune diseases. Of note, unnatural up-regulation of acquired immunity often seen in ST2-deficient mice is not seen in IL-33-deficient mice.73
Since recent large-scale GWAS all show the genes for IL-33 and its receptor are susceptible for asthma, IL-33 now attracts much attention from all investigators involved in asthma research. IL-33 is important for protease-mediated innate airway inflammation and in the late-phase inflammatory responses in the lung observed after IgE-mediated reaction without affecting acquisition of antigen-specific memory T cells. Thus, IL-33 provides a unique therapeutic way for asthma, i.e., ameliorating innate airway inflammation.
Figures and Tables
Fig. 1
Heterodimeric receptors for interleukin-1 (IL-1) family cytokines. IL-1 receptor and IL-33 receptor share IL-1 receptor accessory protein (IL-1RAcP) amplifying the receptor signaling.
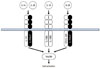
Fig. 2
The major role of interleukin-33 (IL-33) in asthma pathogenesis in comparison with thymic stromal lymphopoietin (TSLP). IL-33 is crucial for protease-mediated innate airway inflammation associated with cell damage, while TSLP profoundly affect the allergen-specific IgE production/Th2 differentiation.
References
1. Oboki K, Ohno T, Kajiwara N, Saito H, Nakae S. IL-33 and IL-33 receptors in host defense and diseases. Allergol Int. 2010. 59:143–160.
2. Onda H, Kasuya H, Takakura K, Hori T, Imaizumi T, Takeuchi T, Inoue I, Takeda J. Identification of genes differentially expressed in canine vasospastic cerebral arteries after subarachnoid hemorrhage. J Cereb Blood Flow Metab. 1999. 19:1279–1288.
3. Baekkevold ES, Roussigné M, Yamanaka T, Johansen FE, Jahnsen FL, Amalric F, Brandtzaeg P, Erard M, Haraldsen G, Girard JP. Molecular characterization of NF-HEV, a nuclear factor preferentially expressed in human high endothelial venules. Am J Pathol. 2003. 163:69–79.
4. Tominaga S. A putative protein of a growth specific cDNA from BALB/c-3T3 cells is highly similar to the extracellular portion of mouse interleukin 1 receptor. FEBS Lett. 1989. 258:301–304.
5. Yanagisawa K, Naito Y, Kuroiwa K, Arai T, Furukawa Y, Tomizuka H, Miura Y, Kasahara T, Tetsuka T, Tominaga S. The expression of ST2 gene in helper T cells and the binding of ST2 protein to myeloma-derived RPMI8226 cells. J Biochem. 1997. 121:95–103.
6. Schmitz J, Owyang A, Oldham E, Song Y, Murphy E, McClanahan TK, Zurawski G, Moshrefi M, Qin J, Li X, Gorman DM, Bazan JF, Kastelein RA. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005. 23:479–490.
7. Ohno T, Oboki K, Kajiwara N, Morii E, Aozasa K, Flavell RA, Okumura K, Saito H, Nakae S. Caspase-1, caspase-8, and calpain are dispensable for IL-33 release by macrophages. J Immunol. 2009. 183:7890–7897.
8. Carriere V, Roussel L, Ortega N, Lacorre DA, Americh L, Aguilar L, Bouche G, Girard JP. IL-33, the IL-1-like cytokine ligand for ST2 receptor, is a chromatin-associated nuclear factor in vivo. Proc Natl Acad Sci U S A. 2007. 104:282–287.
9. Bianchi ME. DAMPs, PAMPs and alarmins: all we need to know about danger. J Leukoc Biol. 2007. 81:1–5.
10. Lotze MT, Tracey KJ. High-mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal. Nat Rev Immunol. 2005. 5:331–342.
11. Lüthi AU, Cullen SP, McNeela EA, Duriez PJ, Afonina IS, Sheridan C, Brumatti G, Taylor RC, Kersse K, Vandenabeele P, Lavelle EC, Martin SJ. Suppression of interleukin-33 bioactivity through proteolysis by apoptotic caspases. Immunity. 2009. 31:84–98.
12. Cayrol C, Girard JP. The IL-1-like cytokine IL-33 is inactivated after maturation by caspase-1. Proc Natl Acad Sci U S A. 2009. 106:9021–9026.
13. Talabot-Ayer D, Lamacchia C, Gabay C, Palmer G. Interleukin-33 is biologically active independently of caspase-1 cleavage. J Biol Chem. 2009. 284:19420–19426.
14. Holgate ST. The airway epithelium is central to the pathogenesis of asthma. Allergol Int. 2008. 57:1–10.
15. Préfontaine D, Nadigel J, Chouiali F, Audusseau S, Semlali A, Chakir J, Martin JG, Hamid Q. Increased IL-33 expression by epithelial cells in bronchial asthma. J Allergy Clin Immunol. 2010. 125:752–754.
16. Küchler AM, Pollheimer J, Balogh J, Sponheim J, Manley L, Sorensen DR, De Angelis PM, Scott H, Haraldsen G. Nuclear interleukin-33 is generally expressed in resting endothelium but rapidly lost upon angiogenic or proinflammatory activation. Am J Pathol. 2008. 173:1229–1242.
17. Liu YJ. Thymic stromal lymphopoietin: master switch for allergic inflammation. J Exp Med. 2006. 203:269–273.
18. Lécart S, Lecointe N, Subramaniam A, Alkan S, Ni D, Chen R, Boulay V, Pène J, Kuroiwa K, Tominaga S, Yssel H. Activated, but not resting human Th2 cells, in contrast to Th1 and T regulatory cells, produce soluble ST2 and express low levels of ST2L at the cell surface. Eur J Immunol. 2002. 32:2979–2987.
19. Löhning M, Stroehmann A, Coyle AJ, Grogan JL, Lin S, Gutierrez-Ramos JC, Levinson D, Radbruch A, Kamradt T. T1/ST2 is preferentially expressed on murine Th2 cells, independent of interleukin 4, interleukin 5, and interleukin 10, and important for Th2 effector function. Proc Natl Acad Sci U S A. 1998. 95:6930–6935.
20. Nakae S, Iwakura Y, Suto H, Galli SJ. Phenotypic differences between Th1 and Th17 cells and negative regulation of Th1 cell differentiation by IL-17. J Leukoc Biol. 2007. 81:1258–1268.
21. Hoshino K, Kashiwamura S, Kuribayashi K, Kodama T, Tsujimura T, Nakanishi K, Matsuyama T, Takeda K, Akira S. The absence of interleukin 1 receptor-related T1/ST2 does not affect T helper cell type 2 development and its effector function. J Exp Med. 1999. 190:1541–1548.
22. Townsend MJ, Fallon PG, Matthews DJ, Jolin HE, McKenzie AN. T1/ST2-deficient mice demonstrate the importance of T1/ST2 in developing primary T helper cell type 2 responses. J Exp Med. 2000. 191:1069–1076.
23. Kondo Y, Yoshimoto T, Yasuda K, Futatsugi-Yumikura S, Morimoto M, Hayashi N, Hoshino T, Fujimoto J, Nakanishi K. Administration of IL-33 induces airway hyperresponsiveness and goblet cell hyperplasia in the lungs in the absence of adaptive immune system. Int Immunol. 2008. 20:791–800.
24. Kurowska-Stolarska M, Kewin P, Murphy G, Russo RC, Stolarski B, Garcia CC, Komai-Koma M, Pitman N, Li Y, Niedbala W, McKenzie AN, Teixeira MM, Liew FY, Xu D. IL-33 induces antigen-specific IL-5+ T cells and promotes allergic-induced airway inflammation independent of IL-4. J Immunol. 2008. 181:4780–4790.
25. Guo L, Wei G, Zhu J, Liao W, Leonard WJ, Zhao K, Paul W. IL-1 family members and STAT activators induce cytokine production by Th2, Th17, and Th1 cells. Proc Natl Acad Sci U S A. 2009. 106:13463–13468.
26. Pecaric-Petkovic T, Didichenko SA, Kaempfer S, Spiegl N, Dahinden CA. Human basophils and eosinophils are the direct target leukocytes of the novel IL-1 family member IL-33. Blood. 2009. 113:1526–1534.
27. Smithgall MD, Comeau MR, Yoon BR, Kaufman D, Armitage R, Smith DE. IL-33 amplifies both Th1- and Th2-type responses through its activity on human basophils, allergen-reactive Th2 cells, iNKT and NK cells. Int Immunol. 2008. 20:1019–1030.
28. Komai-Koma M, Xu D, Li Y, McKenzie AN, McInnes IB, Liew FY. IL-33 is a chemoattractant for human Th2 cells. Eur J Immunol. 2007. 37:2779–2786.
29. Moro K, Yamada T, Tanabe M, Takeuchi T, Ikawa T, Kawamoto H, Furusawa J, Ohtani M, Fujii H, Koyasu S. Innate production of TH2 cytokines by adipose tissue-associated c-Kit+Sca-1+ lymphoid cells. Nature. 2010. 463:540–544.
30. Neill DR, Wong SH, Bellosi A, Flynn RJ, Daly M, Langford TK, Bucks C, Kane CM, Fallon PG, Pannell R, Jolin HE, McKenzie AN. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature. 2010. 464:1367–1370.
31. Spits H, Di Santo JP. The expanding family of innate lymphoid cells: regulators and effectors of immunity and tissue remodeling. Nat Immunol. 2011. 12:21–27.
32. Moritz DR, Rodewald HR, Gheyselinck J, Klemenz R. The IL-1 receptor-related T1 antigen is expressed on immature and mature mast cells and on fetal blood mast cell progenitors. J Immunol. 1998. 161:4866–4874.
33. Chen CC, Grimbaldeston MA, Tsai M, Weissman IL, Galli SJ. Identification of mast cell progenitors in adult mice. Proc Natl Acad Sci U S A. 2005. 102:11408–11413.
34. Iikura M, Suto H, Kajiwara N, Oboki K, Ohno T, Okayama Y, Saito H, Galli SJ, Nakae S. IL-33 can promote survival, adhesion and cytokine production in human mast cells. Lab Invest. 2007. 87:971–978.
35. Allakhverdi Z, Smith DE, Comeau MR, Delespesse G. Cutting edge: the ST2 ligand IL-33 potently activates and drives maturation of human mast cells. J Immunol. 2007. 179:2051–2054.
36. Ho LH, Ohno T, Oboki K, Kajiwara N, Suto H, Iikura M, Okayama Y, Akira S, Saito H, Galli SJ, Nakae S. IL-33 induces IL-13 production by mouse mast cells independently of IgE-FcepsilonRI signals. J Leukoc Biol. 2007. 82:1481–1490.
37. Moulin D, Donzé O, Talabot-Ayer D, Mézin F, Palmer G, Gabay C. Interleukin (IL)-33 induces the release of pro-inflammatory mediators by mast cells. Cytokine. 2007. 40:216–225.
38. Pushparaj PN, Tay HK, H'ng SC, Pitman N, Xu D, McKenzie A, Liew FY, Melendez AJ. The cytokine interleukin-33 mediates anaphylactic shock. Proc Natl Acad Sci U S A. 2009. 106:9773–9778.
39. Min B, Le Gros G, Paul WE. Basophils: a potential liaison between innate and adaptive immunity. Allergol Int. 2006. 55:99–104.
40. Sullivan BM, Locksley RM. Basophils: a nonredundant contributor to host immunity. Immunity. 2009. 30:12–20.
41. Suzukawa M, Iikura M, Koketsu R, Nagase H, Tamura C, Komiya A, Nakae S, Matsushima K, Ohta K, Yamamoto K, Yamaguchi M. An IL-1 cytokine member, IL-33, induces human basophil activation via its ST2 receptor. J Immunol. 2008. 181:5981–5989.
42. Schneider E, Petit-Bertron AF, Bricard R, Levasseur M, Ramadan A, Girard JP, Herbelin A, Dy M. IL-33 activates unprimed murine basophils directly in vitro and induces their in vivo expansion indirectly by promoting hematopoietic growth factor production. J Immunol. 2009. 183:3591–3597.
43. Kroeger KM, Sullivan BM, Locksley RM. IL-18 and IL-33 elicit Th2 cytokines from basophils via a MyD88- and p38-dependent pathway. J Leukoc Biol. 2009. 86:769–778.
44. Cherry WB, Yoon J, Bartemes KR, Iijima K, Kita H. A novel IL-1 family cytokine, IL-33, potently activates human eosinophils. J Allergy Clin Immunol. 2008. 121:1484–1490.
45. Suzukawa M, Koketsu R, Iikura M, Nakae S, Matsumoto K, Nagase H, Saito H, Matsushima K, Ohta K, Yamamoto K, Yamaguchi M. Interleukin-33 enhances adhesion, CD11b expression and survival in human eosinophils. Lab Invest. 2008. 88:1245–1253.
46. Mayuzumi N, Matsushima H, Takashima A. IL-33 promotes DC development in BM culture by triggering GM-CSF production. Eur J Immunol. 2009. 39:3331–3342.
47. Rank MA, Kobayashi T, Kozaki H, Bartemes KR, Squillace DL, Kita H. IL-33-activated dendritic cells induce an atypical TH2-type response. J Allergy Clin Immunol. 2009. 123:1047–1054.
48. Saenz SA, Taylor BC, Artis D. Welcome to the neighborhood: epithelial cell-derived cytokines license innate and adaptive immune responses at mucosal sites. Immunol Rev. 2008. 226:172–190.
49. Soumelis V, Reche PA, Kanzler H, Yuan W, Edward G, Homey B, Gilliet M, Ho S, Antonenko S, Lauerma A, Smith K, Gorman D, Zurawski S, Abrams J, Menon S, McClanahan T, de Waal-Malefyt Rd R, Bazan F, Kastelein RA, Liu YJ. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nat Immunol. 2002. 3:673–680.
50. Ito T, Wang YH, Duramad O, Hori T, Delespesse GJ, Watanabe N, Qin FX, Yao Z, Cao W, Liu YJ. TSLP-activated dendritic cells induce an inflammatory T helper type 2 cell response through OX40 ligand. J Exp Med. 2005. 202:1213–1223.
51. Wang YH, Angkasekwinai P, Lu N, Voo KS, Arima K, Hanabuchi S, Hippe A, Corrigan CJ, Dong C, Homey B, Yao Z, Ying S, Huston DP, Liu YJ. IL-25 augments type 2 immune responses by enhancing the expansion and functions of TSLP-DC-activated Th2 memory cells. J Exp Med. 2007. 204:1837–1847.
52. Omori M, Ziegler S. Induction of IL-4 expression in CD4(+) T cells by thymic stromal lymphopoietin. J Immunol. 2007. 178:1396–1404.
53. Angkasekwinai P, Park H, Wang YH, Wang YH, Chang SH, Corry DB, Liu YJ, Zhu Z, Dong C. Interleukin 25 promotes the initiation of proallergic type 2 responses. J Exp Med. 2007. 204:1509–1517.
54. Yagami A, Orihara K, Morita H, Futamura K, Hashimoto N, Matsumoto K, Saito H, Matsuda A. IL-33 mediates inflammatory responses in human lung tissue cells. J Immunol. 2010. 185:5743–5750.
55. International HapMap Consortium. A second generation human haplotype map of over 3.1 million SNPs. Nature. 2007. 449:851–861.
56. Kim HY, DeKruyff RH, Umetsu DT. The many paths to asthma: phenotype shaped by innate and adaptive immunity. Nat Immunol. 2010. 11:577–584.
57. Gudbjartsson DF, Bjornsdottir US, Halapi E, Helgadottir A, Sulem P, Jonsdottir GM, Thorleifsson G, Helgadottir H, Steinthorsdottir V, Stefansson H, Williams C, Hui J, Beilby J, Warrington NM, James A, Palmer LJ, Koppelman GH, Heinzmann A, Krueger M, Boezen HM, Wheatley A, Altmuller J, Shin HD, Uh ST, Cheong HS, Jonsdottir B, Gislason D, Park CS, Rasmussen LM, Porsbjerg C, Hansen JW, Backer V, Werge T, Janson C, Jönsson UB, Ng MC, Chan J, So WY, Ma R, Shah SH, Granger CB, Quyyumi AA, Levey AI, Vaccarino V, Reilly MP, Rader DJ, Williams MJ, van Rij AM, Jones GT, Trabetti E, Malerba G, Pignatti PF, Boner A, Pescollderungg L, Girelli D, Olivieri O, Martinelli N, Ludviksson BR, Ludviksdottir D, Eyjolfsson GI, Arnar D, Thorgeirsson G, Deichmann K, Thompson PJ, Wjst M, Hall IP, Postma DS, Gislason T, Gulcher J, Kong A, Jonsdottir I, Thorsteinsdottir U, Stefansson K. Sequence variants affecting eosinophil numbers associate with asthma and myocardial infarction. Nat Genet. 2009. 41:342–347.
58. Moffatt MF, Gut IG, Demenais F, Strachan DP, Bouzigon E, Heath S, von Mutius E, Farrall M, Lathrop M, Cookson WO. GABRIEL Consortium. A large-scale, consortium-based genomewide association study of asthma. N Engl J Med. 2010. 363:1211–1221.
59. Melén E, Himes BE, Brehm JM, Boutaoui N, Klanderman BJ, Sylvia JS, Lasky-Su J. Analyses of shared genetic factors between asthma and obesity in children. J Allergy Clin Immunol. 2010. 126:631–637.e8.
60. Reijmerink NE, Postma DS, Bruinenberg M, Nolte IM, Meyers DA, Bleecker ER, Koppelman GH. Association of IL1RL1, IL18R1, and IL18RAP gene cluster polymorphisms with asthma and atopy. J Allergy Clin Immunol. 2008. 122:651–654.e8.
61. Ali M, Zhang G, Thomas WR, McLean CJ, Bizzintino JA, Laing IA, Martin AC, Goldblatt J, Le Souëf PN, Hayden CM. Investigations into the role of ST2 in acute asthma in children. Tissue Antigens. 2009. 73:206–212.
62. Kurowska-Stolarska M, Stolarski B, Kewin P, Murphy G, Corrigan CJ, Ying S, Pitman N, Mirchandani A, Rana B, van Rooijen N, Shepherd M, McSharry C, McInnes IB, Xu D, Liew FY. IL-33 amplifies the polarization of alternatively activated macrophages that contribute to airway inflammation. J Immunol. 2009. 183:6469–6477.
63. Kuroiwa K, Li H, Tago K, Iwahana H, Yanagisawa K, Komatsu N, Oshikawa K, Sugiyama Y, Arai T, Tominaga SI. Construction of ELISA system to quantify human ST2 protein in sera of patients. Hybridoma. 2000. 19:151–159.
64. Oshikawa K, Kuroiwa K, Tago K, Iwahana H, Yanagisawa K, Ohno S, Tominaga SI, Sugiyama Y. Elevated soluble ST2 protein levels in sera of patients with asthma with an acute exacerbation. Am J Respir Crit Care Med. 2001. 164:277–281.
65. Préfontaine D, Lajoie-Kadoch S, Foley S, Audusseau S, Olivenstein R, Halayko AJ, Lemière C, Martin JG, Hamid Q. Increased expression of IL-33 in severe asthma: evidence of expression by airway smooth muscle cells. J Immunol. 2009. 183:5094–5103.
66. Oshikawa K, Yanagisawa K, Tominaga S, Sugiyama Y. Expression and function of the ST2 gene in a murine model of allergic airway inflammation. Clin Exp Allergy. 2002. 32:1520–1526.
67. Hayakawa H, Hayakawa M, Kume A, Tominaga S. Soluble ST2 blocks interleukin-33 signaling in allergic airway inflammation. J Biol Chem. 2007. 282:26369–26380.
68. Mangan NE, Dasvarma A, McKenzie AN, Fallon PG. T1/ST2 expression on Th2 cells negatively regulates allergic pulmonary inflammation. Eur J Immunol. 2007. 37:1302–1312.
69. Meisel C, Bonhagen K, Löhning M, Coyle AJ, Gutierrez-Ramos JC, Radbruch A, Kamradt T. Regulation and function of T1/ST2 expression on CD4+ T cells: induction of type 2 cytokine production by T1/ST2 cross-linking. J Immunol. 2001. 166:3143–3150.
70. Coyle AJ, Lloyd C, Tian J, Nguyen T, Erikkson C, Wang L, Ottoson P, Persson P, Delaney T, Lehar S, Lin S, Poisson L, Meisel C, Kamradt T, Bjerke T, Levinson D, Gutierrez-Ramos JC. Crucial role of the interleukin 1 receptor family member T1/ST2 in T helper cell type 2-mediated lung mucosal immune responses. J Exp Med. 1999. 190:895–902.
71. Liu X, Li M, Wu Y, Zhou Y, Zeng L, Huang T. Anti-IL-33 antibody treatment inhibits airway inflammation in a murine model of allergic asthma. Biochem Biophys Res Commun. 2009. 386:181–185.
72. Cohn L, Homer RJ, Marinov A, Rankin J, Bottomly K. Induction of airway mucus production By T helper 2 (Th2) cells: a critical role for interleukin 4 in cell recruitment but not mucus production. J Exp Med. 1997. 186:1737–1747.
73. Oboki K, Ohno T, Kajiwara N, Arae K, Morita H, Ishii A, Nambu A, Abe T, Kiyonari H, Matsumoto K, Sudo K, Okumura K, Saito H, Nakae S. IL-33 is a crucial amplifier of innate rather than acquired immunity. Proc Natl Acad Sci U S A. 2010. 107:18581–18586.
74. Takai T, Kato T, Hatanaka H, Inui K, Nakazawa T, Ichikawa S, Mitsuishi K, Ogawa H, Okumura K. Modulation of allergenicity of major house dust mite allergens Der f 1 and Der p 1 by interaction with an endogenous ligand. J Immunol. 2009. 183:7958–7965.
75. Humphreys NE, Xu D, Hepworth MR, Liew FY, Grencis RK. IL-33, a potent inducer of adaptive immunity to intestinal nematodes. J Immunol. 2008. 180:2443–2449.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download