Abstract
Purpose
Several studies have proven that EGCG, the primary green tea catechin, and glucosamine-6-phosphate (PGlc) reduce triglyceride contents in 3T3-L1 adipocytes. The objective of this study is to evaluate the combination effect of EGCG and PGlc on decline of accumulated fat in differentiated 3T3-L1 adipocytes. Methods: EGCG and PGlc were administered for 6 day for differentiation of 3T3-L1 adipocytes. Cell viability was measured using the CCK assay kit. In addition, TG accumulation in culture 3T3-L1 adipocytes was investigated by Oil Red O staining. We examined the expression level of several genes and proteins associated with adipogenesis and lipolysis using real-time RT-PCR and Western blot analysis. A flow cytometer Calibar was used to assess the effect of EGCG and PGluco on cell-cycle progression of differentiating 3T3-L1 cells. Results: Intracelluar lipid accumulation was significantly decreased by combination treatment with EGCG 60 μM and PGlc 200 μg/m compared with control and EGCG treatment alone. In addition, use of combination treatment resulted in directly decreased expression of PPARγ, C/EBPα, and SREBP1. In addition, it inhibited adipocyte differentiation and adipogenesis through downstream regulation of adipogenic target genes such as FAS, ACSL1, and LPL, and the inhibitory action of EGCG and PGlc was found to inhibit the mitotic clonal expansion (MCE) process as evidenced by impaired cell cycle entry into S phase and the S to G2/M phase transition of confluent cells and levels of cell cycle regulating proteins such as cyclin A and CDK2. Conclusion: Combination treatment of EGCG and PGlc inhibited adipocyte differentiation through decreased expression of genes related to adipogenesis and adipogenic and cell cycle arrest in early stage of adipocyte differentiation.
Go to : 
References
1. Visscher TL, Seidell JC. The public health impact of obesity. Annu Rev Public Health. 2001; 22:355–375.

2. Lee WJ, Koh EH, Won JC, Kim MS, Park JY, Lee KU. Obesity: the role of hypothalamic AMP-activated protein kinase in body weight regulation. Int J Biochem Cell Biol. 2005; 37(11):2254–2259.

3. Bray GA, Tartaglia LA. Medicinal strategies in the treatment of obesity. Nature. 2000; 404(6778):672–677.

4. Spiegelman BM, Flier JS. Obesity and the regulation of energy balance. Cell. 2001; 104(4):531–543.

5. Ardévol A, Bladé C, Salvadó MJ, Arola L. Changes in lipolysis and hormone-sensitive lipase expression caused by procyanidins in 3T3-L1 adipocytes. Int J Obes Relat Metab Disord. 2000; 24(3):319–324.

6. Balkau B, Valensi P, Eschwège E, Slama G. A review of the metabolic syndrome. Diabetes Metab. 2007; 33(6):405–413.
7. Giri S, Rattan R, Haq E, Khan M, Yasmin R, Won JS, Key L, Singh AK, Singh I. AICAR inhibits adipocyte differentiation in 3T3L1 and restores metabolic alterations in diet-induced obesity mice.
8. Jeon T, Hwang SG, Hirai S, Matsui T, Yano H, Kawada T, Lim BO, Park DK. Red yeast rice extracts suppress adipogenesis by downregulating adipogenic transcription factors and gene expression in 3T3-L1 cells. Life Sci. 2004; 75(26):3195–3203.

9. Chon JW, Sung JH, Hwang EJ, Park YK. Chlorella methanol extract reduces lipid accumulation in and increases the number of apoptotic 3T3-L1 cells. Ann N Y Acad Sci. 2009; 1171:183–189.

10. Qiu Z, Wei Y, Chen N, Jiang M, Wu J, Liao K. DNA synthesis and mitotic clonal expansion is not a required step for 3T3-L1 preadipocyte differentiation into adipocytes. J Biol Chem. 2001; 276(15):11988–11995.

11. Harmon AW, Harp JB. Differential effects of flavonoids on 3T3-L1 adipogenesis and lipolysis. Am J Physiol Cell Physiol. 2001; 280(4):C807–C813.

12. MacDougald OA, Lane MD. Transcriptional regulation of gene expression during adipocyte differentiation. Annu Rev Biochem.
13. Kim SH, Park HS, Lee MS, Cho YJ, Kim YS, Hwang JT, Sung MJ, Kim MS, Kwon DY. Vitisin A inhibits adipocyte differentiation through cell cycle arrest in 3T3-L1 cells. Biochem Biophys Res Commun. 2008; 372(1):108–113.

14. Crespy V, Williamson G. A review of the health effects of green tea catechins in in vivo animal models. J Nutr. 2004; 134(12 Suppl):3431S–3440S.

15. Liao S, Kao YH, Hiipakka RA. Green tea: biochemical and biological basis for health benefits. Vitam Horm. 2001; 62:1–94.

16. Lin J, Della-Fera MA, Baile CA. Green tea polyphenol epigallocatechin gallate inhibits adipogenesis and induces apoptosis in 3T3-L1 adipocytes. Obes Res. 2005; 13(6):982–990.

17. Ahmad N, Mukhtar H. Green tea polyphenols and cancer: biologic mechanisms and practical implications. Nutr Rev. 1999; 57(3):78–83.

18. Kao YH, Hiipakka RA, Liao S. Modulation of endocrine systems and food intake by green tea epigallocatechin gallate. Endocrinology. 2000; 141(3):980–987.

19. Nagao T, Komine Y, Soga S, Meguro S, Hase T, Tanaka Y, Tokimitsu I. Ingestion of a tea rich in catechins leads to a reduction in body fat and malondialdehyde-modified LDL in men. Am J Clin Nutr. 2005; 81(1):122–129.

20. Kim JD, Lee BI, Jeon YH, Bak JP, Jin HL, Lim BO. Anti-oxidative and antiinflammatory effects of green tea mixture and dietary fiber on liver of high fat diet-induced obese rats. Korean J.
21. Graham HN. Green tea composition, consumption, and polyphenol chemistry. Prev Med. 1992; 21(3):334–350.

22. Chan CY, Wei L, Castro-Muñozledo F, Koo WL. (−)-Epigallocat-echin-3-gallate blocks 3T3-L1 adipose conversion by inhibition of cell proliferation and suppression of adipose phenotype expression. Life Sci. 2011; 89(21–22):779–785.

23. Nagao T, Meguro S, Hase T, Otsuka K, Komikado M, Tokimitsu I, Yamamoto T, Yamamoto K. A catechin-rich beverage improves obesity and blood glucose control in patients with type 2 diabetes. Obesity (Silver Spring). 2009; 17(2):310–317.

24. Kim SO, Lee HE, Choe WK. The effects of ginseng saponin-Re, Rc and green tea catechine; ECGC (Epigallocatechin gallate) on leptin, hormone sensitive lipase and resistin mRNA expressions in 3T3-L1 adipocytes. Korean J Nutr. 2006; 39(8):748–755.
25. Moon HS, Lee HG, Choi YJ, Kim TG, Cho CS. Proposed mechanisms of (−)-epigallocatechin-3-gallate for anti-obesity. Chem Biol Interact. 2007; 167(2):85–98.

26. Lee CS. Health functional food technology and research status in domestic and foreign. Bull Food Technol. 2005; 18(4):38–54.
27. Kong CS, Kim JA, Kim SK. Anti-obesity effect of sulfated glucosamine by AMPK signal pathway in 3T3-L1 adipocytes. Food Chem Toxicol. 2009; 47(10):2401–2406.

28. Kong CS, Kim JA, Eom TK, Kim SK. Phosphorylated glucosamine inhibits adipogenesis in 3T3-L1 adipocytes. J Nutr Biochem. 2010; 21(5):438–443.

29. Kim MM, Mendis E, Rajapakse N, Kim SK. Glucosamine sulfate promotes osteoblastic differentiation of MG-63 cells via antiinflammatory effect. Bioorg Med Chem Lett. 2007; 17(7):1938–1942.

30. Gregoire FM, Smas CM, Sul HS. Understanding adipocyte differentiation. Physiol Rev. 1998; 78(3):783–809.

31. Tang QQ, Lane MD. Activation and centromeric localization of CCAAT/enhancer-binding proteins during the mitotic clonal expansion of adipocyte differentiation. Genes Dev. 1999; 13(17):2231–2241.

32. Kim H, Sakamoto K. (−)-Epigallocatechin gallate suppresses adipocyte differentiation through the MEK/ERK and PI3K/Akt pathways. Cell Biol Int. 2012; 36(2):147–153.

33. Sung HY, Hong CG, Suh YS, Cho HC, Park JH, Bae JH, Park WK, Han J, Song DK. Role of (−)-epigallocatechin-3-gallate in cell viability, lipogenesis, and retinol-binding protein 4 expression in adipocytes. Naunyn Schmiedebergs Arch Pharmacol. 2010; 382(4):303–310.

34. Latasa MJ, Moon YS, Kim KH, Sul HS. Nutritional regulation of the fatty acid synthase promoter in vivo: sterol regulatory element binding protein functions through an upstream region containing a sterol regulatory element. Proc Natl Acad Sci U S A. 2000; 97(19):10619–10624.

35. Luong A, Hannah VC, Brown MS, Goldstein JL. Molecular characterization of human acetyl-CoA synthetase, an enzyme regulated by sterol regulatory element-binding proteins. J Biol Chem. 2000; 275(34):26458–26466.

36. Rosen ED. The transcriptional basis of adipocyte development. Prostaglandins Leukot Essent Fatty Acids. 2005; 73(1):31–34.

37. Rosen ED, Walkey CJ, Puigserver P, Spiegelman BM. Transcriptional regulation of adipogenesis. Genes Dev. 2000; 14(11):1293–1307.

38. Palmer DG, Rutter GA, Tavaré JM. Insulin-stimulated fatty acid synthase gene expression does not require increased sterol response element binding protein 1 transcription in primary adipocytes. Biochem Biophys Res Commun. 2002; 291(3):439–443.

39. Bulló M, García-Lorda P, Peinado-Onsurbe J, Hernández M, Del Castillo D, Argilés JM, Salas-Salvadó J. TNFalpha expression of subcutaneous adipose tissue in obese and morbid obese females: relationship to adipocyte LPL activity and leptin synthesis. Int J Obes Relat Metab Disord. 2002; 26(5):652–658.
40. Kim SK, Kong CS. Anti-adipogenic effect of dioxinodehydroeck-ol via AMPK activation in 3T3-L1 adipocytes. Chem Biol Interact. 2010; 186(1):24–29.

41. Lee MS, Kim CT, Kim IH, Kim Y. Inhibitory effects of green tea catechin on the lipid accumulation in 3T3-L1 adipocytes. Phytother Res. 2009; 23(8):1088–1091.

42. Reichert M, Eick D. Analysis of cell cycle arrest in adipocyte differentiation. Oncogene. 1999; 18(2):459–466.

43. Kim CY, Le TT, Chen C, Cheng JX, Kim KH. Curcumin inhibits adipocyte differentiation through modulation of mitotic clonal expansion. J Nutr Biochem. 2011; 22(10):910–920.

44. Li X, Kim JW, Gr⊘nborg M, Urlaub H, Lane MD, Tang QQ. Role of cdk2 in the sequential phosphorylation/activation of C/EBP-beta during adipocyte differentiation. Proc Natl Acad Sci U S A. 2007; 104(28):11597–11602.

45. Kim SI, Park CS, Lee MS, Kwon MS, Jho EH, Song WK. Cyclin-dependent kinase 2 regulates the interaction of Axin with beta-catenin. Biochem Biophys Res Commun. 2004; 317(2):478–483.
46. Auld CA, Fernandes KM, Morrison RF. Skp2-mediated p27 (Kip1) degradation during S/G2 phase progression of adipocyte hyperplasia. J Cell Physiol. 2007; 211(1):101–111.
Go to : 
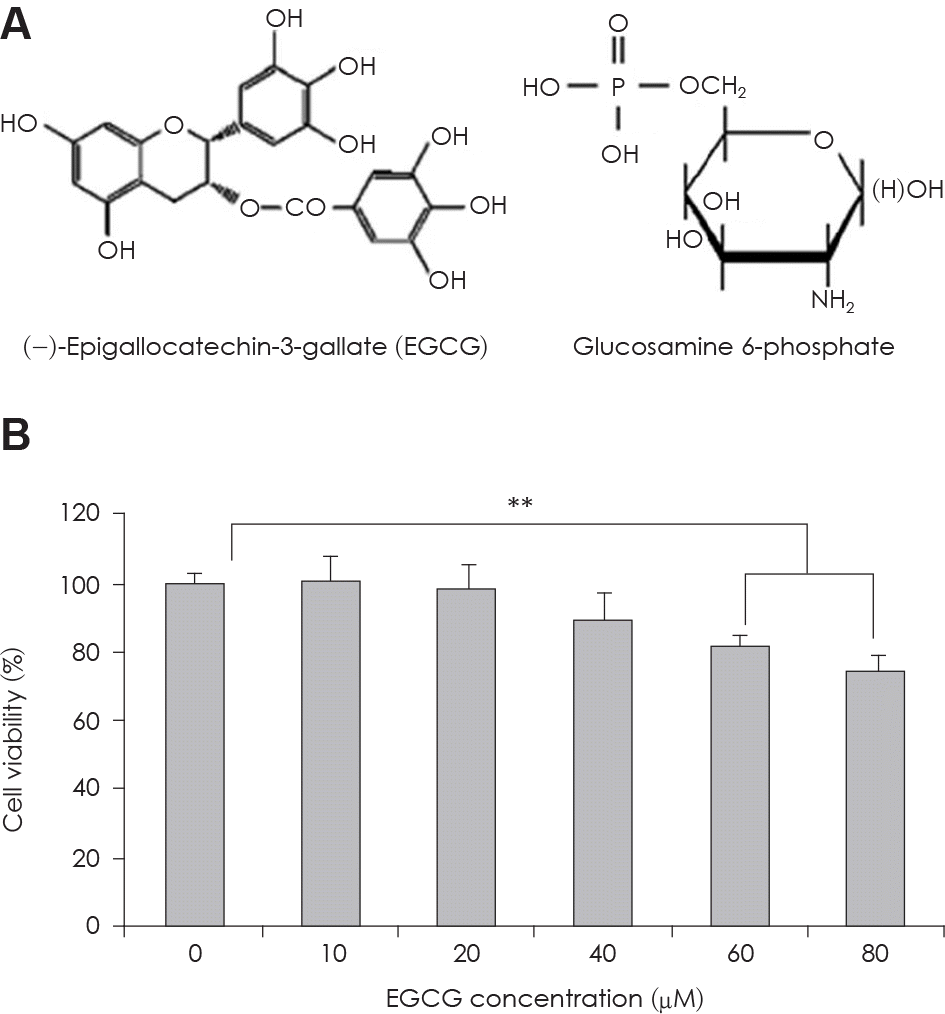 | Fig. 1.A: Structure of EGCG and Glucosamine 6-phosphate. B: The change of cell viability in 3T3-L1 preadipocyte in condition of EGCG of serial concentrations. Value represent the mean ± SEM. ∗∗: p < 0.001, vs. EGCG 0 μM. |
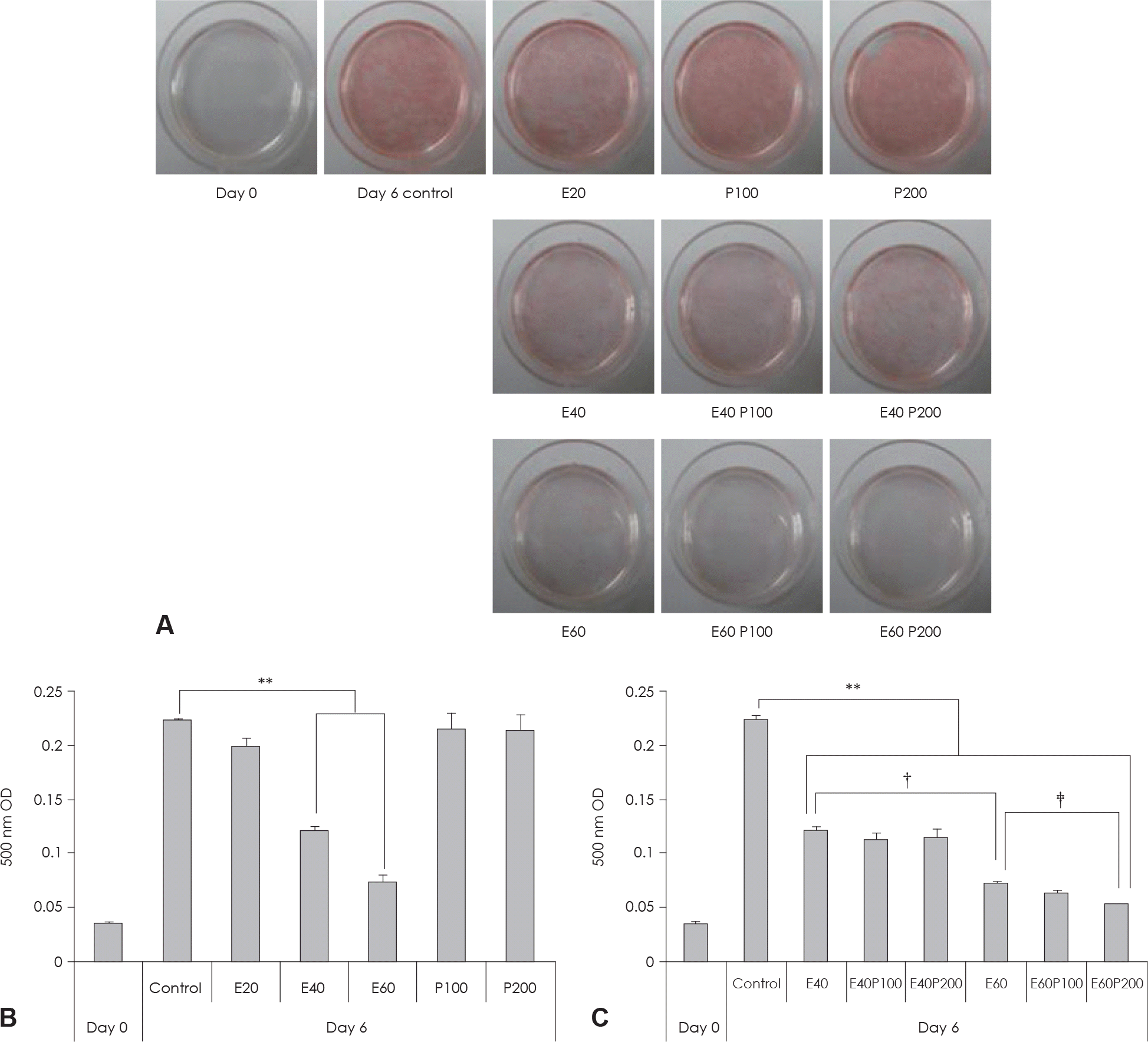 | Fig. 2.Effect of EGCG & Glucosamine 6-phosphate on lipid accumulation in 3T3-L1 cells. A: Cell were subjected to Oil Red O staining B, C: These cells were then subjected to quantitative analysis of interacelluar lipid accumulation. Value represent the mean ± SEM. ∗∗: p < 0.001, vs. control. †: p < 0.05, E40 vs. E60. ‡: p < 0.05, E60 vs. E60P200. E: EGCG, P: Glucosamine 6-phosphate. |
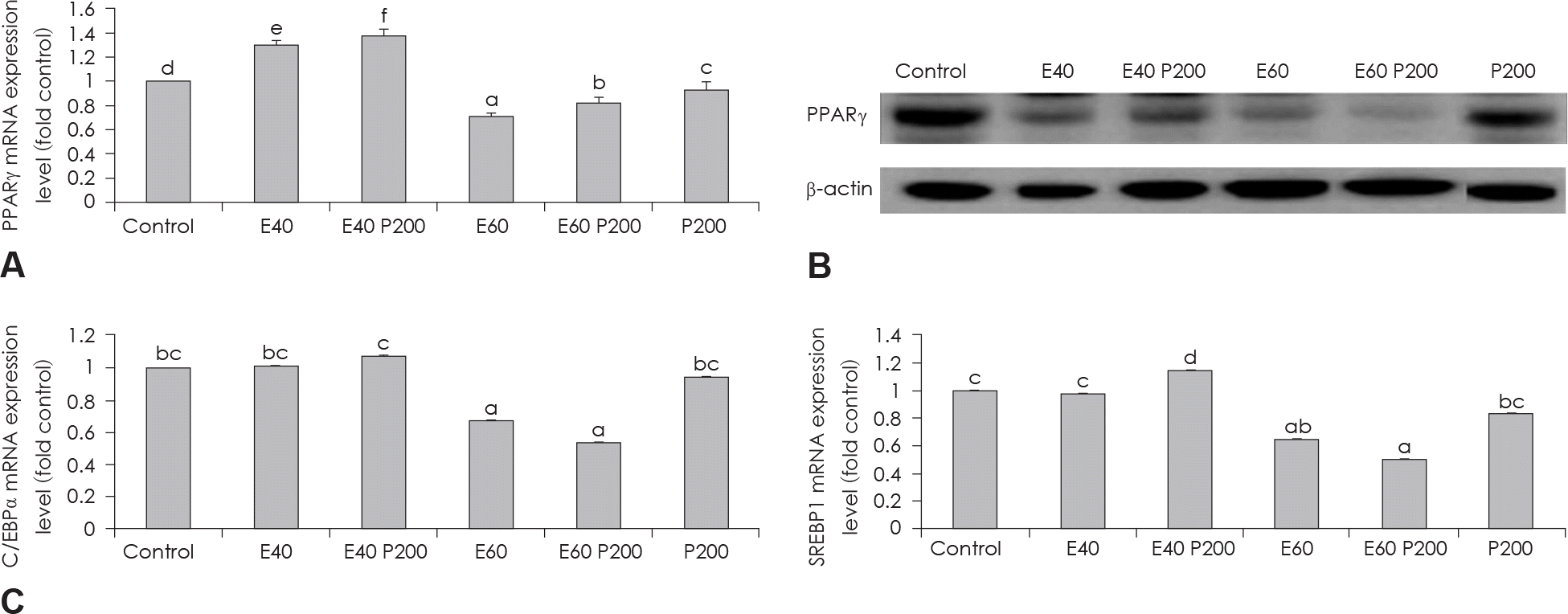 | Fig. 3.Effect of EGCG & Glucosamine 6-phosphate on adipogenesis transcription factors. PPARγ expression in 3T3-L1 cells as examined by real time PCR (A) and western blot (B) analysis. C/EBPα and SREBP1 expression in 3T3-L1 cells as examined by real time PCR (C). Confluent 3T3-L1 preadipocytes in medium with or without differentiation concentrations of GCG & Glucosamine 6-phosphate for 6 days (from day 0 to day 6) were differentiated into adipocytes. Value represent the mean ± SEM. Means with different letters (a-f) at each mRNA are significantly different (p < 0.05) by Duncan's multiple range test. Control: fully differentiated control adipocytes, E: with EGCG, P: Glucosamine 6-phosphate. |
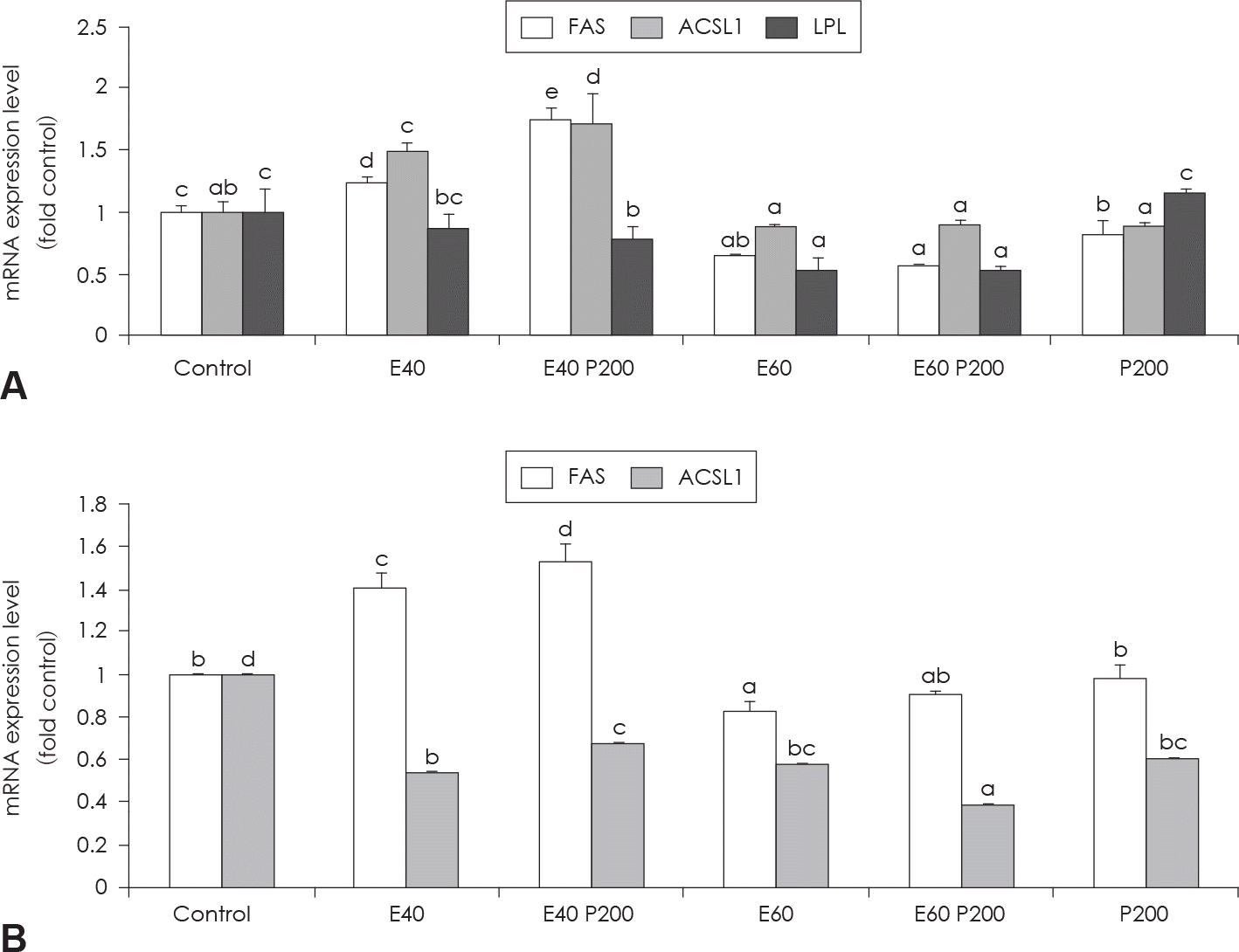 | Fig. 4.Effect of EGCG & Glucosamine 6-phosphate on FAS, ACSL1 and LPL expression (A) and HSL and perilipin expression (B) as examined by real time PCR analysis. Confluent 3T3-L1 preadipocytes in medium with or without differentiation concentrations of GCG & Glucosamine 6-phosphate for 6 days (from day 0 to day 6) were differentiated into adipocytes. Value represent the mean ± SEM. Means with different letters (a-d) at each mRNA are significantly different (p < 0.05) by Duncan's multiple range test. Control: fully differentiated control adipocytes, E: with EGCG, P: Glucosamine 6-phosphate |
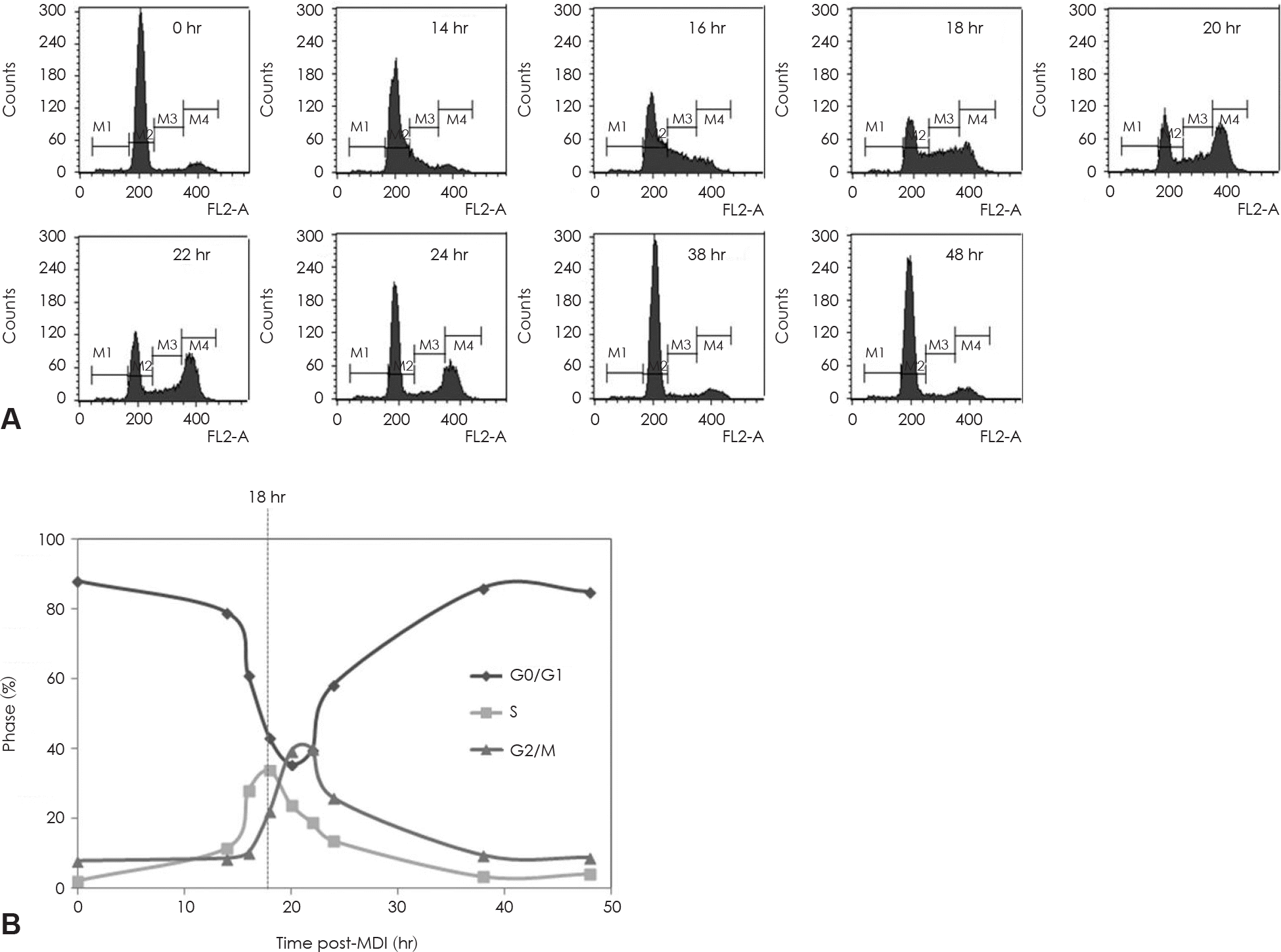 | Fig. 5.Mitotic clonal expansion during the 3T3-L1 preadipocyte differentiation induction process. 3T3-L1 preadipocyte differentiation was induced with the standard induction protocol. FACS analysis for DNA content of 3T3-L1 preadipocytes during mitotic clonal ex-panssion after the differentiation induction. A: Stained with PI for DNA histograms generated with flow cytomatric analysis. B: Cell cycle phase distribution plotted over time follow in MDI exposure. Dotted lines through phase intersections approximate the kinetics of phase transitions. M1: subG1 phase, M2: G0/G1 phase, M3: S phase, M4: G2/M phase. |
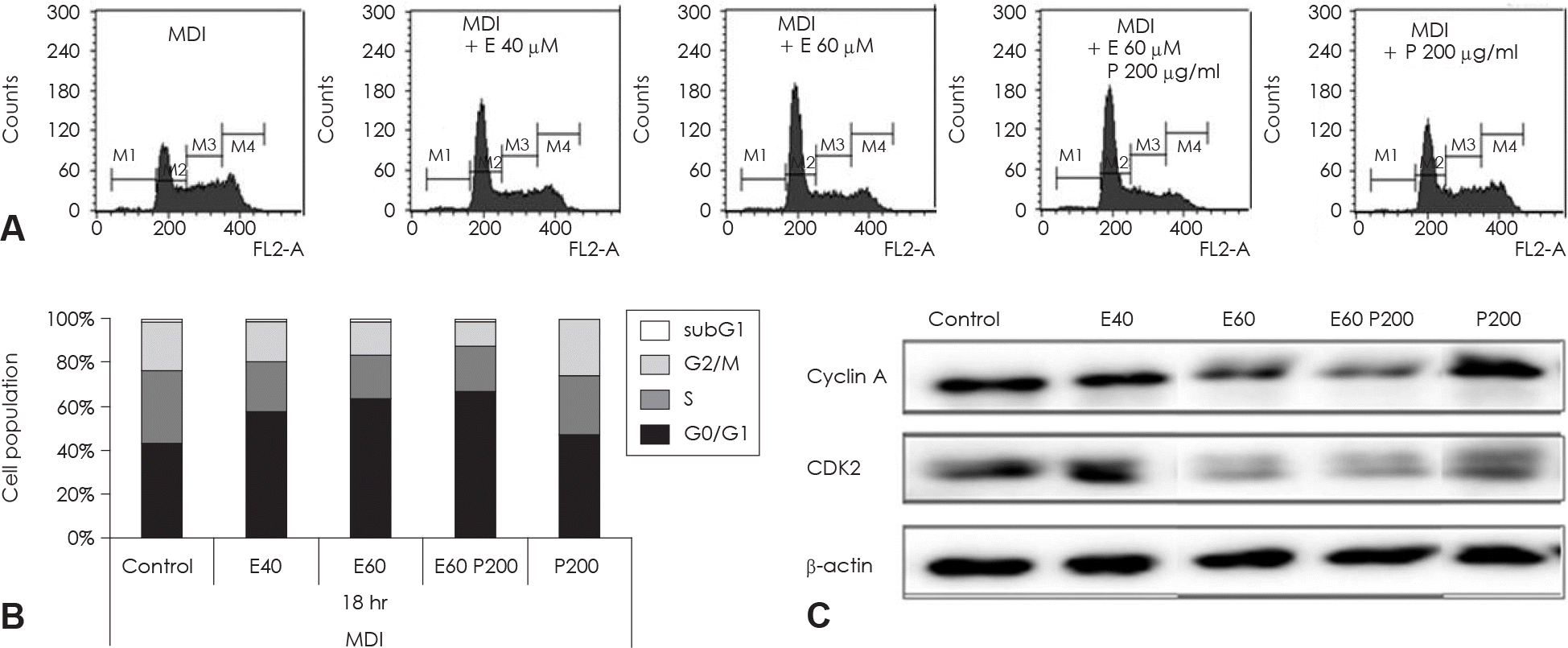 | Fig. 6.Cell cycle analysis of EGCG and glucosamine-6-phosphate treated 3T3-L1 cell during th MCE process of adipocyte differentiation. Two day postconfluent 3T3-L1 cell were indicated with the adipogenic cocktail in the presence or absence of various concentration and alone or combination of EGCG and glucosamine-6-phosphate. After 18hr of treatment, differentiating cell were stained with propidium iodine (PI). These cells were then subjected to flow cytometric cell-cycle analysis (A) and quantitative analysis of cells in different phases in cell cycle (B). Data were analyzed using CellQuest Pro software. (C) The protein levels of cyclinA and CDK2 in these cells were detected by immunoblot assay using their specific antibody, β-actin was used as a loading control. M1: subG1 phase, M2: G0/G1 phase, M3: S phase, M4: G2/M phase. Control: fully differentiated control adipocytes, P: with Glucosamine 6-phosphate. |
Table 1.
Primer sequences used for RT-PCR




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download