INTRODUCTION
Seaweeds are categorized as brown algae, red algae, and green algae, and domestic seaweeds contain 6–42% of proteins and 6–74% of carbohydrates with some variation [
12]. Sea algae also contain a variety of bioactive substances such as polyphenols, polysaccharides, minerals and amino acids. Especially, red algae are well-known for their biological activities including antibacterial, anti-oxidant, and anti-asthmatic effects [
3456]. Red algae are composed of approximately 40–75% carbohydrate based on their dry weight, and the majority components are cellulose, xylan, mannan, and agar [
7]. Among them,
Gelidium amansii (
GA) contains plenty of agars, which make them a popular functional food for reducing body weight. Also,
GA has been reported to have various bioactive properties such as antioxidant, anti-inflammatory, anti-diabetic, anti-obesity and anti-atherogenic effects [
8910].
A recent study reported a decrease of paraepididymal and perirenal fat tissues, blood triglyceride, and inflammatory cytokines such as adipocytokines, tumor necrosis factor (TNF)-α and interleukin (IL)-6 in rats fed high-fat diet (HFD) +
GA for 11 wks [
11]. Also, agar consumption to diabetic patients to reduce hunger sensation had a cholesterol-lowering effect as well as reducing blood glucose levels and fat mass [
12].
GA extract reduced mRNA expression of NADPH oxidase 4 (NOX4) and a reactive oxygen species (ROS) marker while it increased antioxidant enzymes protein expressions such as superoxide dismutase (SOD) 1/2, glutathione peroxidase (GPx), and glutathione reductase (GR) in fully differentiated 3T3-L1 adipocytes [
10].
Previously, other studies have demonstrated an anti-inflammatory effect of
GA-ethanol extracts in
in vitro model by using mouse preadipocytes or
in vivo mouse model [
81314]. Collectively, various studies have shown that agar in
GA may have anti-obesity effects; however, it is not clear yet whether the bioactive properties of
GA extract (
GAE) are due to agar itself or not. In this study, we investigated potential health-beneficial effects of
GAE without agar,
AfGAE, in diet-induced obese (DIO) C57BL/6J mouse model. Initially, anti-obesity effects of
AfGAE were examined in HFD-induced obese mouse model as a pilot study. With anti-inflammatory and lipolytic effects of
GAE without agar observed in the pilot study, further studies were carried out to compare the preventive model vs treatable model with various doses of
GAE without agar in C57BL/6J mice.
DISCUSSION
Prevalence of obesity has been increasing continuously, and the same phenomenon is true in South Korea, which exhibits 150% increased prevalence of obesity compared to that of a decade ago [
18]. Obesity-induced chronic inflammation causes macrophage infiltration in adipose tissue and various inflammatory cytokine productions which consequently are involved in insulin resistance, type 2 diabetes, and cardiovascular diseases [
192021]. Therefore, it has been an active research area to screen functional foods and/or compounds that have an anti-obesity property.
GA,
Gelidium amansii, containing plenty of agar is known to have anti-obesity, antioxidant, and anticarcinogenic effects [
9111213]. Thus, agar itself has been recognized as active principles used to treat obesity [
22], whereas other potential bioactive compounds in
GA were not studied intensively. In this current study, we therefore investigated the effects of agar-free GA extract (
AfGAE) by using diet-induced obese C57BL/6J mouse model. First, a pilot study was carried out to evaluate effects of
AfGAE in a diet-induced obese mouse model. In addition, to find out an optimal concentration and proper approach strategy of
AfGAE for potential health beneficial effects in DIO model, we further studied effects of various doses of
AfGAE by using preventive and treatment of
AfGAE against obesity and its associated harmful consequences in an
in vivo model.
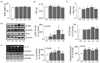
AfGAE administration in the pilot experiment did not prevent HFD induced obesity in C57BL/6J mice, but appeared to significantly increase IL-10 levels in epididymal adipose tissue compared to control group. Furthermore, an increase of HSL phosphorylation was observed by AfGAE administration to HFD fed C57BL/6M mice. Thus, AfGAE administration affected an increase of IL-10 expression in epididymal adipose tissue; suggesting the anti-inflammatory effects of AfGAE may not be due to agar itself in C57BL/6J mice. Collectively, besides agar, other components in the AfGAE may play a role to regulate the anti-inflammatory effects that shall warrant future studies.
Unlike previous studies conducted in DIO mouse model with
GA containing agar, this present study did not show anti-obesity effects of
AfGAE suggesting that agar might be the responsible element for the anti-obesity effect of
GA. Kang et al. [
8] and Yang et al. [
11] reported the inhibitory effects of
GA with agar on adiposity in mice and rats, respectively. Kang et al. [
8] showed the anti-obesity effects of
Gelidium amansii ethanol-extracts in high-fat diet (45% calories from fat) fed C57BL/6 mice for 12 wks. Yang et al. [
11] demonstrated that minced
GA feeding improves plasma glucose and lipid profiles in high-fat diet fed streptozotocin nicotinamide-induced diabetic rats. A recent study by Kang et al. [
23] also demonstrated the anti-obesity effect of
GA extracts in obese mice that fed only a high fat diet (60% calories from fat) for 5 wks which was a similar experimental design as our Exp-2, a treatment model. Although there are various factors such as sample
GA preparation method and solvents of
GA (ethanol extract of
GA vs. minced
GA), types of animal models (drug-induced diabetic model vs. diet-induced obesity model), types of high-fat diet used (45% calories from fat vs. 60% calories from fat), duration of feeding periods and when to introduce
GA, GAE administration indeed prevented and/or treated high-fat diet induced obesity in mice [
823]. Furthermore, Maeda et al. [
7] demonstrated that agar diet reduced body weight by maintaining reduced calorie intake in obese patients with impaired glucose tolerance and type 2 diabetes. However, those patients were also instructed moderated intensity exercise along with fixed portions of agar prepared in tasty ways. Thus, anti-obesity effects of
GA observed in three animal models and human subjects mentioned above suggest that agar in
GA plays a critical role to regulate weight management despite a difficulty to compare them directly.
Adiponectin is known to act as a positive regulator to improve insulin resistance and inflammation. For example, a decrease of TNF-α by inhibiting NF-kB caused an increase in adiponectin levels in rodents [
24]. In this present study, adiponectin levels in mesenteric adipose tissue of mice fed HFD +
AfGAE was significantly increased compared to that of mice fed HFD in a preventive model,
Exp-1 study. Alteration of adiponectin levels by
AfGAE administration, however, was not observed in a treatment model,
Exp-2. This discrepancy of results between
Exp-1 and
Exp-2 could be explained by the following; (1) timing to start
AfGAE administration, (2) tissue-specificity of certain cytokine productions, and (3) different sensitivity of certain cytokine production by local inflammation vs. systemic inflammation due to aging.
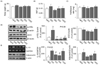
AfGAE used in this present study, was hydrolyzed to remove agar. During the hydrolyzation process, reducing sugar contents in
GA increased by 110% [
1]. It showed that increased reducing sugar contents in
AfGAE did not have impacts on anti-obesity effects in HFD-fed C57BL/6J mice. HSL, a lipase induced by either fasting or catecholamine secretion, mobilize the stored fats by hydrolyzing the first fatty acid from a triglyceride molecule, freeing a fatty acid and diglyceride [
25]. A recent study reported a decrease of paraepididymal and perirenal fat tissues, blood triglyceride, and inflammatory cytokines such as adipocytokines, TNF-α and IL-6 in rats fed HFD + GA for 11 wks [
11]. Furthermore, Park et al. demonstrated that fucoid, brown algae, induced lipolysis by enhancing HSL phosphorylation [
26]. In the current study, HSL phosphorylation in mesenteric fat was significantly increased by
AfGAE in a dose-dependent manner compared to that of mice fed HFD only in a prevention study,
Exp-1 (
P < 0.001), whereas this dose-dependency phenomenon was not observed in a treatment study,
Exp-2. It is worthwhile to note that the preventive method for
AfGAE might be a better way to approach the potential health benefits on lipolysis related factors.
Comparing
GA with agar and without agar (
AfGAE), the anti-inflammatory effects of
GAE with or without agar might be not due to agar itself, but other bioactive compound(s) in them. Thus, it is assumed that other active compounds such as polyphenols of
GAE besides agar may play an important role in regulating obesity [
27]. However, the responsible compounds related to various potential health-beneficial effects of
GAE are not yet clear. Therefore, it is suggested that further studies should be performed on the isolation and identification of the component in
GA. Strengths of this study are (1) conducting a series of experiments step by step to investigate the potential effects of
AfGAE, and (2) finding out a better approach for
AfGAE to have its superior effects, whereas limitations of this study are (1) not being able to show the responsible compound in
AfGAE for anti-inflammatory effect, and (2) only measuring the local anti-inflammation,
IL-10 and adiponectin in abdominal fat pads not the systemic (i.e., plasma) inflammation.
Collectively the current study demonstrated that AfGAE enhanced anti-inflammatory cytokine production in a diet-induced obese mouse model despite being obese. In addition, we could conclude that the anti-inflammatory effect is independent of agar in GA. Although future studies are required, the result suggests that GA may be a therapeutic tool to improve health conditions related to inflammation.







 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download