Abstract
In this study, antioxidant and free radical scavenging activities of the natural antioxidative compound, pyrogallol-phloroglucinol-6,6'-bieckol (PPB) isolated from brown algae, Ecklonia cava was assessed in vitro by measuring the radical scavenging activities (DPPH, alkyl, hydroxyl, and superoxide) using electron spin resonance (ESR) spectrometry, intracellular reactive oxygen species (ROS) scavenging activity, and DNA damage assay. According to the results of these experiments, the scavenging activity PPB against difference radicals was in the following order: DPPH, alkyl, hydroxyl, and superoxide radicals (IC50; 0.90, 2.54, 62.93 and 109.05 µM). The antioxidant activities of PPB were higher than that of the commercial antioxidant, ascorbic acid. Furthermore, PPB effectively inhibited DNA damage induced by H2O2. These results suggest that the natural antioxidative compound, PPB, can be used by the natural food industry.
Reactive oxygen species (ROS) including superoxide anion radical, hydroxyl radical, alkyl radical and hydrogen peroxide, which are formed and degraded by all aerobic organism, can cause oxidative damage to all major groups of biomolecules (DNA, protein, lipids and small cellular molecules), and has been shown to lead to cardiovascular and neurodegenerative diseases [1]. Antioxidants can inhibit or delay the initiation or propagation of the oxidative chain reaction and thus prevent the repair of cell damage caused by ROS. Accordingly, synthetic antioxidants, such as butylated hydroxytoluene (BHT) and butylated hydroxyanisole (BHA), have been extensively used as additives for oxidation suppressant in foods, cosmetics and drug compositions. However, the possible toxicity as well as general consumer rejection of synthetic antioxidants has led to a decrease in the use of these compounds [2]. Therefore, research on natural antioxidants has recently been on the rise. Many studies have been carried out to find new antioxidant compounds from natural resources such as plants [3], foods [4] and marine algae [5,6]. Especially, foods phytochemicals such as phenolic compounds have potential protective effects against many diseases [7]. Therefore, consumption of a variety of phenolic compounds with high antioxidant effects may reduce the risk of serious health disorders caused by ROS.
Ecklonia cava, which is a brown alga that is present in high quantities on Jeju Island of Korea, is not available in Europe. E. cava is also popular in Korea and Japan, where these valuable brown algae are utilized in food ingredients, animal feeds, fertilizers and medicines [8]. The results of recent studies have shown that E. cava displays strong antioxidant activity. However, further studies will be needed to determine and isolate the natural antioxidative compoundss from E. cava.
The objectives of the present study were to isolate and evaluate the antioxidant properties of compounds from E. cava.
Silica gel 60 (0.036-0.2 mm Merck), Celite®545, TLC plates (Kieselgel 60 F254 5715, Merck), 5,5-Dimethyl-1-pyrrolin N-oxide (DMPO), 2,2-azobis (2-amidinopropane) hydrochloride (AAPH), a-(4-pyridyl-1-oxide)-N-t-butylnitrone (4-POBN), 1,1-diphenyl-2-picrylhydrazyl (DPPH), FeSO4, and hydrogen peroxide; for the cell culture experiment reagents, 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), 2'7'-dichlorodihydrofluorescein diacetate (DCFH-DA) were acquired from Sigma Chemical Co. (St. Louis, Mo, USA). All other reagents were of analytical grade.
The brown alga, E. cava, was collected along the coast of Jeju Island, Korea, between February and May 2010. The samples were washed three times in tap water to remove any attached salt, epiphytes, and sand, then rinsed carefully with fresh distilled water, and maintained in a medical refrigerator at -20℃. The frozen samples were then lyophilized and homogenized using a grinder prior to extraction.
The dried E. cava powder (500 g) was extracted using 5 L of 80% aqueous methanol three times at room temperature. The liquid layer was obtained via filtration, and the filtrate was concentrated using an evaporator under reduced pressure. The extract was suspended in H2O, and the aqueous layer was partitioned with ethyl acetate (EtOAc). The EtOAc extract (45.65 g) was mixed with celite. The mixed celite was then dried and packed into a glass column, and subsequently eluted in the following order: hexane, dichloromethane, diethyl ether, and butanol. The diethyl ether fraction (26.69 g) was subjected to Silica column chromatography using a stepwise gradient with chloroform/methanol (2/1→0/1) to yield subfractions (E. cava-diethyl ether fraction, ECE) based on TLC analysis (Fig. 1).
HPLC-ESI-MS analysis of ECE was conducted using an LXQ system (Thermo Fisher Scientific, USA) equipped with a 2.1 mm × 100 mm i.d., 1.9 µm particle size, Hypersil-Gold C18 column. The sample was then separated for 40 min using a gradient mobile phase consisting of 10% to 100% methanol. The flow rate was set at 200 µL/min and the injection volume was 2 µL. The detection wavelength was set to 230 nm. Positive ionization mode was applied in a full scan range of 500-1,000. The optimized electrospray operation conditions were as follows: capillary voltage 5 kV, temperature 275℃, and sheath gas 30 mL/min. HPLC separation of ECE7 was conducted using a Dionex system (Dionex, USA) equipped with a 19 mm × 300 mm i.d., 10 µm particle size, µBondapak™ C18 column. The same chromatographic conditions as described above were employed, except the flow rate was set to 5 mL/min.
NMR spectra were recorded using a JNM ECP-400 spectrometer (JNM ECP-400, JEOL, Japan), operated at 500 MHz for 1H NMR and 100 MHz for 13C NMR. The isolated active compound was prepared in deuterated solvents in 5mm NMR tubes. The deuterated solvent employed was DMSO-d6 and the chemical shifts were measured relative to the TMS signal. All experiments were conducted at room temperature.
The different radicals tested here were generated according to previously described procedures [5], and the spin adducts were recorded using a JES-FA electron spin resonance (ESR) spectrometer (JES-FA ESR, JEOL, Japan).
The DPPH radical scavenging activity was evaluated using an ESR spectrometer in accordance with the method described by Nanjo et al. [9]. A 60 µL sample was added to 60 µL of DPPH (60 µM) in ethanol. After 10 seconds of vigorous mixing, the solution was transferred to Teflon capillary tubes and fitted into the cavity of the ESR spectrometer exactly 2 min later, under the following measurement conditions: central field 3475 G, modulation frequency 100 kHz, modulation amplitude 2 G, microwave power 5 mW, gain 6.3 × 105, and temperature 25℃. All radical scavenging activities (%) were calculated using the following equation, where H and H0 were the relative peak heights of the radical signals with and without PPB, respectively.
% radical scavenging activity = [1-(H / H0)] × 100
Alkyl radicals were generated via AAPH. The reaction mixture containing 10 mM AAPH and 10 mM 4-POBN were mixed with PPB. The solution was incubated for 30 min at 37℃ in a water bath, and then transferred to Teflon capillary tubes [10]. The spin adduct was recorded using a JES-FA ESR spectrometer under the following measurement conditions: central field 3475 G, modulation frequency 100 kHz, modulation amplitude 2 G, microwave power 10 mW, gain 6.3 × 105, and temperature 25℃. The radical scavenging activity (%) was presented as described in section 3.1.
Hydroxyl radicals were generated via a Fenton reaction, and rapidly reacted with a DMPO nitrone spin trap. The resultants DMPO-OH adducts were detected using an ESR spectrometer [11]. Reaction mixtures containing 100 µL of 0.3 M DMPO, 100 µL of 10 mM FeSO4, and 100 µL of 10 mM H2O2 were mixed with PPB, and transferred to Teflon capillary tubes. The spin adducts were measured using an ESR spectrometer exactly 2.5 min later, under the following measurement conditions: central field 3475 G, modulation frequency 100 kHz, modulation amplitude 2 G, microwave power 1 mW, gain 6.3 × 105, and temperature 25℃. The hydroxyl radical scavenging activity (%) was presented as described in section 3.1.
Superoxide radicals were generated via the UV irradiation of a riboflavin/EDTA system [12]. The reaction mixture containing 0.3 mM riboflavin, 1.6 mM EDTA, 800 mM DMPO, and the indicated concentrations of PPB were irradiated for 1 min under a UV lamp at 365 nm. The reaction mixture was transferred to a 100 µL Teflon capillary tube in the ESR spectrometer for measurement. Experimental conditions were as follows: magnetic field 336.5 ± 5 mT, power 10 mW, modulation frequency 9.41 GHz, amplitude 1 × 1000, sweep time 1 min, gain 6.3 × 105, and temperature 25℃. The radical scavenging activity (%) is shown as described in section 3.1.
The monkey kidney fibroblast cell line (Vero) was maintained at 37℃ in an incubator with a humidified atmosphere of 5% CO2. The cells were cultured in Dulbecco's modified Eagle's medium containing 10% heat-inactivated fetal bovine serum, streptomycin (100 µg/mL), and penicillin (100 unit/mL).
Cell cytotoxicity was estimated via the MTT assay, which is a test of metabolic competence predicated based on mitochondrial performance. It is a colorimetric assay, which depends on the conversion of yellow tetrazolium bromide to its purple formazan derivative via mitochondrial succinate dehydrogenase in viable cells [13]. Vero cells were seeded in 96-well plates at a concentration of 1 × 105 cells/mL. After 16 hr, the cells were treated with PPB at different concentrations for 24 hr at 37℃. The MTT stock solution (50 µL; 2 mg/mL) was then applied to the wells to a total reaction volume of 250 µL. After 4 hr of incubation, the plates were centrifuged for 5 min at 800 × g, and the supernatants were aspirated. The formazan crystals in each well were dissolved in 150 µL of dimethylsulfoxide (DMSO), and the absorbance was measured via ELISA at a wavelength of 540 nm. Relative cell cytotoxicity was evaluated according to the quantity of MTT converted to insoluble formazan salt. The optical density of the formazan generated in the control cells was considered to represent 100% viability. The data were expressed as the mean percentage of the viable cells versus the respective control.
For the detection of intracellular ROS, Vero cells were seeded in 96-well plates at a concentration of 1 × 105 cells/mL. After 16 hr, the cells were treated with various concentrations of PPB and incubated at 37℃ under a humidified atmosphere. After 30 min, H2O2 was added at a final concentration of 1 mM, and the cells were incubated for an additional 30 min at 37℃. Finally, 2',7'-dichlorodihydrofluorescein diacetate (DCFH-DA; 5 µg/mL) was introduced to the wells, and DCFH fluorescence was detected at an excitation wavelength of 485 nm and an emission wavelength of 535 nm, using a Perkin-Elmer LS-5B spectrofluorometer. The percentage of intracellular ROS scavenging activity was calculated using the following equation:
Intracellular ROS scavenging activity (%) = (1-(C1 / C0)) × 100
in which C1 is the fluorescence intensity of cells treated with H2O2 and compound, and C0 is the fluorescence intensity of cells treated with only H2O2.
The alkaline comet assay was conducted according to the method described by Ahn et al. [14]. The number of cultured cells was adjusted to 1 × 105 cells/mL, and the cells were incubated with PPB at concentrations ranging from 25 to 100 µg/mL, which were selected based on the results of the hydrogen peroxide scavenging activity measurements, for 30 min at 37℃ in a dark incubator. After preincubation, the cells were centrifuged at minimum rpm for 5 min and washed with phosphate buffer saline (PBS). The cells were then resuspended in PBS with 50 µM H2O2 for 5 min on ice. The untreated control cells were resuspended in PBS only, without H2O2. The cells were then washed with 1 mL PBS and centrifuged. The cell suspensions were mixed with 100 µL of 0.7% low melting point agarose (LMPA) at 37℃ and spread on a fully frosted microscopic slide that was pre-coated with 100 µL of 1% normal melting point agarose (NMPA). After the solidification of the agarose, the slide was covered with another 100 µL of 0.7% LMPA and subsequently immersed in a lysis solution (2.5 M NaCl, 100 mM Na-EDTA, 10 mM Tris, 1% Trion X-100, and 10% DMSO, pH 10) for 1 hr at 4℃. The slides were then placed in a buffer containing 300 mM NaOH and 10 mM Na-EDTA (pH 13) for 20 min to allow DNA unwinding and to measure the alkali labile damage. An electrical field was applied (300 mA, 25 V) for 20 min at 4℃ to draw negatively charged DNA toward the anode. After electrophoresis, the slides were washed three times with neutralizing buffer (0.4 M Tris, pH 7.5) for 5 min at 4℃ and stained with 50 µL of ethidium bromide (20 µg/mL). The slides were observed using a fluorescence microscope and the result images were analyzed (Kinetic Imaging, Komet 5.5, UK). The percentage of total fluorescence in the tail and the tail length of the 50 cells/slide were recorded.
All the data were expressed as mean ± standard deviation (SD) of three determinations. Statistical comparison was performed via one-way analysis of variance (ANOVA) followed by Duncan's multiple range test (DMRT). P-values of less than 0.05 (P < 0.05) were considered significant.
E. cava is present in abundance along the coast of Jeju Island, and is generally regarded as a useful material for the prevention or treatment of several human diseases. Since there have been relatively few biological studies conducted with this alga, here, we attempted to detect and isolate a novel antioxidative compound from the alga.
In this study, we attempted to assess the antioxidant effects of the MeOH extract derived from E. cava along with its solvent soluble fractions, including n-hexane, dichlorometahne, diethyl ether, n-buthanol, and H2O layer at concentrations of 100 µg/mL (data not shown). Among the partitioned fractions of the methanol extract, the diethyl ether fraction (64.1%) displayed noticeable DPPH radical scavenging activity. Therefore, we conducted further experiments and isolated the active compound of the diethyl ether fraction via repeated column chromatography over silica gel and RP-18 gel. The structure of the active compound was determined by 1D (1H and 13C NMR) spectroscopic analyses and comparison to published data [15]. The molecular ion in the negative ESI-MS of the active compound at m/z 973 corresponded to the molecular formula of C48H30O23. The chemical shifts observed in the 1H and 13C NMR experiments indicated that the polyphenolic structure was phlorotannin, since resonance signals were in the range of δ 5.8-6.3 and δ 94-162. The 1H NMR data indicated the presence of fifteen aromatic protons and fifteen phenolic hydroxyl protons. The 13C NMR signals corresponded to fifteen non-substituted and thirty three O-bearing aromatic carbons. This data suggested that the active compound contained eight units of phloroglucinol. The NMR spectral data were similar to those of 6, 6'-bieckol [16]. However, when compared to 6, 6'-bieckol, the chemical shift of C-5''' (δ 159.3) was up field. This suggested that two additional units of phloroglucinol were connected with C-5'''. The chemical shifts of C-1'''' (δ 154.0) C-4'''' (δ 122.5) and C-1''''' (δ 159.9) and HMBC correlations between H-2''''/H-6'''' and C-1''''/C-4'''', H-2'''''/H-6''''' and C-1''''' indicated the presence of two ether linkages between three rings. The 1H and 13C NMR data of the active compound are listed in Fig. 2, and shown in Table 1 and 2. Based on the analysis of these spectral data, the active compound was deduced and tentatively reported to be pyrogallol-phloroglucinol-6,6'-bieckol (PPB).
ESR spin trapping is a sensitive, direct, and accurate method to monitor reactive species [12]. Therefore, in this study ESR, was employed to evaluate the DPPH, alkyl, hydroxyl, and superoxide radical scavenging activities of PPB isolated from E. cava, and compare these activities with that of a commercial antioxidant, ascorbic acid.
DPPH has been widely used to test the ability of compounds to act as free radical scavengers or hydrogen donors, and to evaluate the antioxidant activity of foods. Therefore, DPPH was used to evaluate the antioxidative activity of PPB. The ESR spectra of different concentrations of PPB in the presence of DPPH are shown in Fig. 3A. It was noted that the ESR signals of PPB were attenuated when the concentrations increased from 0.25 to 5 µM. PPB had a higher IC50 value for DPPH radical scavenging activity (IC50 = 0.90 µM) than that of the commercial antioxidant, ascorbic acid (IC50 = 19.92 µM) (Table 3), and the radical scavenging activity was found to be dose-dependent.
The alkyl radical spin adduct was observed when AAPH was incubated for 30 min with the spin trap 4-POBN at 37℃, and these radicals were measured via ESR. The ability of PPB to scavenge alkyl radicals is presented in Fig. 3B. As shown in this figure, 63.62% of the alkyl radicals were scavenged by PPB at a concentration of 6.25 µM. The attenuation of the ESR signal was observed with an increase in the PPB concentration; hence, the activity was determined to be dose-dependent. Furthermore, PPB had a higher IC50 value for alkyl radical scavenging (IC50 = 2.54 µM) than that of ascorbic acid (IC50 = 19.19 µM) (Table 3). Therefore, this result indicates that PPB possesses remarkable scavenging ability for alkyl radicals.
Hydroxyl radicals are an extremely reactive oxygen species, capable of modifying almost every molecule in the living cells. This radical has the capacity to cause strand damage in DNA, which can lead to carcinogenesis, mutagenesis, and cytotoxicity. Moreover, hydroxyl radicals are capable of quick initiation of the lipid peroxidation process by abstracting hydrogen atoms from unsaturated fatty acids [17]. The hydroxyl radical scavenging activities of PPB are shown in Fig. 3C. As shown in this figure, a decrease in the amount of DMPO-OH adducts was observed after the addition of PPB, and radical scavenging activity was dose-dependent. The PPB displayed strong hydroxyl radical scavenging activity and higher IC50 values (IC50 = 62.93 µM) than ascorbic acid (IC50 = 69.66 µM) (Table 3), and the radical scavenging activity was found to be dose-dependent.
Superoxide anion radicals play a major role in the formation of other reactive oxygen species, such as hydroxyl radical, hydrogen peroxide and single oxygen in living systems. Superoxide radicals can induce aging and destroy the cell membrane, and can be generated by oxidative stress. The ESR signals and the scavenging activity of PPB against superoxide radicals are shown in Fig. 3D. As shown in this figure, PPB showed almost the same activity (IC50 = 109.05 µM) as the commercial antioxidant (IC50 = 98.45 µM) (Table 3). Based on these results, this novel antioxidant compound, PPB, appears to function as an electron donor that is capable of neutralizing free radicals. Consequently, PPB is a potential radical scavenging agent that suppresses oxidation in humans and in food.
The survival rates of cells were higher than 95% at all of concentrations tested (6.25 to 100 µM), which indicate that this compound produced no cytotoxic effects in Vero cells (Fig. 4).
The direct scavenging effects of PPB on cellular radicals were investigated in order to confirm their ability to scavenge free radicals in cellular environments. To determine whether PPB could prevent H2O2-induced ROS generation and the resultant oxidative stresses, levels of ROS production in the cells were determined using the fluorescence probe DCF. In this study, we attempted to evaluate the antioxidant effects of PPB in Vero cells after H2O2 treatment. The intracellular ROS scavenging activity of the compound was shown in Fig. 5. As shown in this figure, 68.79% of intracellular ROS was scavenged at a concentration of 5 µM, and this activity was dose-dependent. PPB had an IC50 value for intracellular ROS scavenging (IC50 = 3.54 µM) than the values obtained for ascorbic acid.
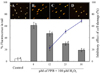
The protective effects of PPB on cell damage were also verified via comet assays (Fig. 6). Photomicrographs of different DNA migration profiles upon treatment with different PPB concentrations are provided in Fig. 6. In cells exposed to only H2O2, the DNA was completely damaged, but the addition of PPB during H2O2 treatment resulted in a dose-dependent suppression of DNA damage. In fact, PPB had a DNA damage-inhibitory activity of 54.9% even at a concentration of 25 µM (IC50 value was 23.2 µM).
Recent developments in biomedical science have emphasized the involvement of free radicals in many diseases, such as brain dysfunction, cancer, heart disease and immune system [1]. Dietary antioxidant intake may be an important strategy for inhibiting or delaying the oxidation of susceptible cellular substrates, and is thus relevant to disease prevention in many paradigms. Polyphenolic compounds, such as flavonoids, phenolic acids, and tannins, have received attention in this regard due to their high antioxidative activity. Fruit, vegetable, and algae, and other plant materials rich in polyphenolic components are of increasing interest in the food industry because they can retard oxidative degradation of lipids and improve the quality and nutritional value of food [18,19]. Polyphenolic compounds that are known to possess high antioxidative activity are common phytochemicals in fruits and leafy vegetables. Previous studies have shown that there was a direct relationship between antioxidant activity and phenolic compounds in herbs, vegetables and fruits [20-22].
Since there are a large number of different types of antioxidant compounds that might contribute to the total antioxidant capacity, it is not clear which components are responsible for the observed antioxidant activity. To examine the effect of the phytochemical constituents of E. cava on antioxidant capacity, we determined the correlation between the antioxidant capacity and main antioxidant substances, polyphenolic compounds. The antioxidant capacity of E. cava appeared to be largely affected by the levels of polyphenolic compounds and the structure-activity relationship.
Many studies have suggested that the radical scavenging activities of polyphenols such as flavonoids are due to the phenolic hydroxyl groups attached to the benzene ring, and the structure-activity relationship of flavonoids has been examined using a wide range of different antioxidant assays [23,24]. Although the structure-activity relationship of polyphenol is not clear, it is plausible that the phenolic hydroxyl groups attached to the eckol skeleton play an important role in the radical scavenging activities [25]. In the present study, the radical scavenging activities of PPB was much higher than that of phloroglucinol, which implied that the amount of hydroxyl groups attached to the benzene ring indeed played an important role in the radical scavenging activities [6].
In conclusion, our results demonstrated that the novel polyphenol compound isolated from E. cava, PPB, displayed different degrees of potency in its radical scavenging and protective effects against H2O2-induced damage in Vero cells. Therefore, based on our results, PPB may prove to be a useful natural radical scavenger and a potential supplement for use by the food, pharmaceutical, and cosmetic industries, due to its profound antioxidant properties.
Figures and Tables
Fig. 2
MS spectroscopic and chemical structure of the active compound isolated from E. cava. The spectra were generated in negative ionization mode (A). Chemical structure and HMBC correlation of the active compound isolated from E. cava (B).
Fig. 3
Free radical scavenging activities of PPB measured using an ESR spectrometer. (A) DPPH radical; (B) Alkyl radical; (C) Hydroxyl radical; (D) Superoxide radical. Experiments were conducted in triplicate and the data were expressed as the means ± SE. Values with different alphabets are significantly different at P < 0.05 as analyzed by Duncan's multiple range test (DMRT).

Fig. 4
The cytotoxic effect of the PPB isolated from E. cava at different concentrations. The viability of Vero cells was determined using the MTT assay. Experiments were conducted in triplicate and the data are expressed as the means ± SE. Values with different alphabets are significantly different at P < 0.05 as analyzed by Duncan's multiple range test (DMRT).

Fig. 5
Effect of the PPB on scavenging intracellular reactive oxygen species. The intracellular reactive oxygen species generated were detected via the DCFH-DA method. Experiments were conducted in triplicate and the data were expressed as the means ± SE. Values with different alphabets are significantly different at P < 0.05 as analyzed by Duncan's multiple range test (DMRT).

Fig. 6
Protective effect of different concentrations of the PPB on H2O2-induced DNA damage using the comet assay. Photomicrographs of DNA damage and migration observed under PPB. (A) Control; (B) 100 µM H2O2; (C) 25 µg/mL of PPB + 100 µM H2O2; (D) 50 µg/mL of PPB + 100 %M H2O2. The cells damaged by H2O2-treatment were assessed via the comet assay. Experiments were conducted in triplicate and the data were expressed as the means ± SE. Values with different alphabets are significantly different at P < 0.05 as analyzed by Duncan's multiple range test (DMRT).
References
1. Halliwell B, Gutteridge JMC. Free Radicals in Biology and Medicine. 1999. 3rd ed. New York: Oxford University Press;1–35.
2. Branen AL. Toxicology and biochemistry of butylated hydroxyanisole and butylated hydroxytoluene. J Am Oil Chem Soc. 1975. 52:59–63.

3. Choi CW, Kim SC, Hwang SS, Choi BK, Ahn HJ, Lee MY, Park SH, Kim SK. Antioxidant activity and free radical scavenging capacity between Korean medicinal plants and flavonoids by assay-guided comparison. Plant Sci. 2002. 163:1161–1168.

4. Borneo R, León AE, Aguirre A, Ribotta P, Cantero JJ. Antioxidant capacity of medicinal plants from the province of cordoba (argentina) and their in vitro testing in a model food system. Food Chem. 2009. 112:664–670.

5. Heo SJ, Kim JP, Jung WK, Lee NH, Kang HS, Jun EM, Park SH, Kang SM, Lee YJ, Park PJ, Jeon YJ. Identification of chemical structure and free radical scavenging activity of diphlorethohydroxycarmalol isolated from a brown alga, Ishige okamurae. J Microbiol Biotechnol. 2008. 18:676–681.
6. Zou Y, Qian ZJ, Li Y, Kim MM, Lee SH, Kim SK. Antioxidant effects of phlorotannins isolated from Ishige okamurae in free radical mediated oxidative systems. J Agric Food Chem. 2008. 56:7001–7009.

7. Rice-Evans CA, Sampson J, Bramley PM, Holloway DE. Why do we expect carotenoids to be antioxidants in vivo? Free Radic Res. 1997. 26:381–398.
8. Guiry MD, Blunden G. Seaweed Resources in Europe: Uses and Potential. 1991. New York: John Wiley & Sons Ltd.;335–351.
9. Nanjo F, Goto K, Seto R, Suzuki M, Sakai M, Hara Y. Scavenging effects of tea catechins and their derivatives on 1,1-diphenyl-2-picrylhydrazyl radical. Free Radic Biol Med. 1996. 21:895–902.

10. Hiramoto K, Johkoh H, Sako K, Kikugawa K. DNA breaking activity of the carbon-centered radical generated from 2,2'-azobis (2-amidinopropane) hydrochloride (AAPH). Free Radic Res Commun. 1993. 19:323–332.

11. Rosen GM, Rauckman EJ. Packer L, editor. Spin trapping of superoxide and hydroxyl radicals. Methods in Enzymology. 1984. New York: Academic Press;198–209.
12. Guo Q, Zhao B, Shen S, Hou J, Hu J, Xin W. ESR study on the structure-antioxidant activity relationship of tea catechins and their epimers. Biochim Biophys Acta. 1999. 1427:13–23.

13. Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983. 65:55–63.

14. Ahn GN, Kim KN, Cha SH, Song CB, Lee J, Heo MS, Yeo IK, Lee NH, Jee YH, Kim JS, Heu MS, Jeon YJ. Antioxidant activities of phlorotannins purified from Ecklonia cava on free radical scavenging using ESR and H2O2-mediated DNA damage. Eur Food Res Technol. 2007. 226:71–79.

15. Kang HS, Chung HY, Jung JH, Son BW, Choi JS. A new phlorotannin from the brown alga Ecklonia stolonifera. Chem Pharm Bull (Tokyo). 2003. 51:1012–1014.
16. Artan M, Li Y, Karadeniz F, Lee SH, Kim MM, Kim SK. Anti-HIV-1 activity of phloroglucinol derivative, 6,6'-bieckol, from Ecklonia cava. Bioorg Med Chem. 2008. 16:7921–7926.

17. Kappus H. Aruoma OI, Halliwell B, editors. Lipid peroxidation: Mechanism and biological relevance. Free Radicals and Food Additives. 1991. New York: Talyor and Francis;59–75.
18. Senevirathne M, Kim SH, Jeon YJ. Protective effect of enzymatic hydrolysates from highbush blueberry (Vaccinium corymbosum L.) against hydrogen peroxide-induced oxidative damage in Chinese hamster lung fibroblast cell line. Nutr Res Pract. 2010. 4:183–190.

19. Shukla S, Mehta A, John J, Singh S, Mehta P, Vyas SP. Antioxidant activity and total phenolic content of ethanolic extract of Caesalpinia bonducella seeds. Food Chem Toxicol. 2009. 47:1848–1851.

20. Sun J, Yao J, Huang S, Long X, Wang J, García-García E. Antioxidant activity of polyphenol and anthocyanin extracts from fruits of Kadsura coccinea (Lem.) A.C. Smith. Food Chem. 2009. 117:276–281.

21. Chew YL, Goh JK, Lim YY. Assessment of in vitro antioxidant capacity and polyphenolic composition of selected medicinal herbs from Leguminosae family in Peninsular Malaysia. Food Chem. 2009. 116:13–18.

22. Faller ALK, Fialho E. The antioxidant capacity and polyphenol content of organic and conventional retail vegetables after domestic cooking. Food Res Int. 2009. 42:210–215.

23. Burda S, Oleszek W. Antioxidant and antiradical activities of flavonoids. J Agric Food Chem. 2001. 49:2774–2779.





 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download