Abstract
Dapsone, an antibiotic, has been used to cure leprosy. It has been reported that dapsone has anti-inflammatory activity in hosts; however, the anti-inflammatory mechanism of dapsone has not been fully elucidated. The present study investigated the anti-inflammatory effects of dapsone on bone marrow cells (BMs), especially upon exposure to lipopolysaccharide (LPS). We treated BMs with LPS and dapsone, and the treated cells underwent cellular activity assay, flow cytometry analysis, cytokine production assessment, and reactive oxygen species assay. LPS distinctly activated BMs with several characteristics including high cellular activity, granulocyte changes, and tumor necrosis factor alpha (TNF-α) production increases. Interestingly, dapsone modulated the inflammatory cells, including granulocytes in LPS-treated BMs, by inducing cell death. While the percentage of Gr-1 positive cells was 57% in control cells, LPS increased that to 75%, and LPS plus dapsone decreased it to 64%. Furthermore, dapsone decreased the mitochondrial membrane potential of LPS-treated BMs. At a low concentration (25 µg/mL), dapsone significantly decreased the production of TNF-α in LPS-treated BMs by 54%. This study confirmed that dapsone has anti-inflammatory effects on LPS-mediated inflammation via modulation of the number and function of inflammatory cells, providing new and useful information for clinicians and researchers.
Dapsone is a member of the sulfone family and has been used as an antibiotic [16]. To inhibit the growth of bacteria, dapsone disturbs the synthesis of dihydrofolic acid in bacteria by competing with para-aminobenzoic acid by acting on dihydropteroate synthetase [1617]. Dapsone has been used to treat multibacillary leprosy in combination with rifampicin and clofazimine [17].
Dapsone has been shown to have anti-inflammatory effects in addition to antibiotic activity, and two functional mechanisms have been suggested so far. The first is to modulate the production of inflammatory cytokines. Dapsone inhibits the secretion of interleukin-8 from lipopolysaccharide (LPS)-stimulated human bronchial epithelial cells [8] and the production of tumor necrosis factor alpha (TNF-α) from activated mononuclear cells [1]. The second mechanism suggested for the anti-inflammatory effect of dapsone is to inhibit myeloperoxidase (MPO) in neutrophils. MPO in neutrophils produces hypohalous acids involved in the microbicidal activity of neutrophils [3]. A previous study demonstrated that dapsone affected the function of neutrophils by blocking MPO in azurophilic granules; as a result, dapsone reduced the accumulation of hypohalous acid, a causative agent for tissue damage [9]. The effect of dapsone on neutrophil MPO is associated with its anti-inflammatory effects. Although these two mechanisms of dapsone have been studied, the anti-inflammatory effects of dapsone have not been fully elucidated yet.
In this study, we treated mouse bone marrow cells (BMs) with LPS to simulate the inflammatory reaction environment in osteomyelitis. LPS acts as prototypical endotoxin in tissues and binds to various receptors including cluster of differentiation 14 and Toll-like receptor 4 in many types of immune cells such as macrophages [12]. The binding of LPS triggers the production of pro-inflammatory cytokines, nitric oxide, and eicosanoids [14].
In the present study, we cultured mouse BMs in the presence of LPS and after treatment with dapsone. The levels of cellular activity, cytokine production, and reactive oxygen species (ROS) were analyzed in the study experiments.
C57BL/6 mice were purchased from OrientBio (Korea) and maintained in our animal facility. The present study used 8- to 12-week-old mice. All experiments using animal were performed according to the institutional guidelines of Jeju National University for laboratory animal use and care (IACUC No. 2015-0017). Dapsone and LPS purified from Escherichia coli O55 were purchased from Sigma (USA). For use, dapsone and LPS were dissolved in sterile phosphate buffered saline.
BMs were harvested from the femurs and tibias of C57BL/6 mice as described previously [10]. Any contaminated red blood cells in the samples were eliminated by applying ACK lysis buffer. BMs were filtered through a 70 µm cell strainer to obtain single cells. To culture BMs, RPMI1640 media containing 5% fetal bovine serum, 2 mM L-glutamine, and 100 U/mL penicillin/streptomycin (Thermo Fisher Scientific, USA) were used.
The cellular activity of BMs was evaluated after treatment. Briefly, BMs were seeded at a concentration of 1 × 106 cells/mL in 96-well culture plates and treated with LPS and varying concentrations of dapsone. After culturing for 3 days, 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT; Sigma) was added for 4 h (0.5 mg/mL) [7]. The viable cells generated insoluble violet crystals, and 100 µL/well of 10% sodium dodecyl sulfate solution was added for 2 h to dissolve the crystals. The optical density of the sample was measured at 570 nm by using a microplate reader (Multiskan FC; Thermo Fisher Scientific).
To observe alterations of cell morphology, BMs were seeded at a concentration of 1 × 106 cells/mL in 24-well culture plates and then treated with LPS and dapsone. After culturing for 3 days, the morphology of treated cells was photographed using an inverted microscope (IX70; Olympus, Japan) and a digital camera.
BMs (1 × 106 cells/mL) were established in 96-well culture plates and treated with LPS and dapsone at the indicated concentration. After 3 days, the supernatants were collected and used to determine the level of TNF-α, a representative pro-inflammatory cytokine. The cytokine concentration was measured by performing an enzyme-linked immunosorbent assay (ELISA) using a CytoSet antibody pair (Thermo Fisher Scientific) based on the manufacturer's manual.
BMs were cultured for 3 days at a concentration of 1 × 106 cells/mL in 6-well culture plates and treated with 50 µg/mL dapsone and 1 µg/mL LPS. For flow cytometry analysis, the treated BMs were harvested and subjected to multiple assays. We measured cell size (forward scatter [FSC]) and cell granularity (side scatter [SSC]) of the treated BMs. To analyze cell death, both apoptosis and necrosis, the cells were stained with 0.25 µg/mL propidium iodide (PI; Sigma). To check the mitochondrial membrane potential (MMP) in the BMs, the cells were incubated with 10 µg/mL Rhodamine 123 (Sigma) for 30 min at room temperature. Additionally, to detect granulocytes, a specific antibody for granulocyte differentiation antigen 1 (Gr-1) was used as the primary antibody with streptavidin-fluorescein isothiocyanate as the secondary antibody (BD Biosciences, USA). To measure intracellular ROS level, the cells were treated with 10 µM dichlorofluorescein diacetate (DCFDA; Sigma). All stained cells were analyzed by using a FACSCalibur flow cytometer and CellQuest software (BD Biosciences).
The data from the MTT assay and ELISA are presented as mean ± SD values and were statistically analyzed by one-way analysis of variance followed by Tukey's multiple comparison test as provided by InStat (GraphPad Software, USA). A p value of < 0.05 was considered significant. The *, **, and *** symbols indicate p < 0.05, 0.01, and 0.001, respectively, compared to control values.
To investigate the effects of dapsone on LPS-treated BMs, the cells were treated with LPS at 1 µg/mL and dapsone over a range of concentrations (0–100 µg/mL). Results of MTT assays demonstrated that LPS significantly enhanced the cellular activity of BMs compared to that in control BMs without LPS treatment (Fig. 1). Dapsone significantly decreased the cellular activity of LPS-treated BMs across a range of dapsone concentrations (12.5–100 µg/mL). These results indicate that dapsone can suppress the cellular activity of BMs that have been stimulated by inflammatory agents, such as LPS. In the following experiments (Figs. 2, 3, 4 and panel B in Fig. 5), the 50 µg/mL concentration of dapsone was used based on the drug's function and cytotoxicity.
In the dot plots of panel A in Fig. 2, the region circled includes granulocytes, such as neutrophils, based on the relationship between FSC and SSC. LPS increased the percentage of the granulocytes, and dapsone markedly decreased the LPS increase in granulocytes. This result indicates that dapsone considerably decreases the number of activated granulocytes in an LPS-induced inflammation state. To further investigate if the region within the dot plots includes granulocytes, we employed Gr-1 antigen to detect granulocytes specifically. The BMs were stained with anti-Gr-1 antibody and plotted against SSC (panel B in Fig. 2). While the percentage of Gr-1 positive cells was 57% in control cells, LPS increased that level to 75%, while dapsone plus LPS decreased to 64%. The dot plots confirmed that LPS increases the percentage of granulocytes with enhanced granularity (SSC), whereas dapsone decreases the LPS-increased percentage of granulocytes. In panel B in Fig. 2, two regions can be detected based on the extent of the granularity (i.e., middle and high SSC, R1 and R2, respectively). Interestingly, dapsone decreased the percentage of granulocytes in R1 (middle SSC) in the LPS-treated BMs (panel B in Fig. 2).
To observe the morphological changes in BMs produced by LPS and/or dapsone treatment, we obtained pictures by using an inverted microscope connected to a digital camera. There were no notable differences between the control and dapsone-treated BMs (Fig. 3). However, LPS treatment generated some markedly large BMs (Fig. 3; black arrows) after 3 days of treatment, and dapsone treatment decreased the size of the large BMs in LPS-treated BMs.
To investigate how dapsone affects LPS-activated BMs, we measured MMP of the cells. For this purpose, a rhodamine 123 solution was used to stain BMs treated with LPS and/or dapsone. LPS enhanced the MMP of BMs, whereas dapsone profoundly decreased the LPS-increased MMP of BMs (panel A in Fig. 4). This indicates that dapsone can destabilize the structure of the mitochondrial double membrane. To investigate further the cellular status, PI solution was used to detect the percentage of dead cells. It is generally accepted that the cell size of dead or dying cells is gradually decreased. Thus, we defined the dot plot region with low FSC and high PI as one contains dead cells (panel B in Fig. 4). LPS decreased the percentage of dead cells from that in the control samples. However, dapsone increased the percentage of dead cells compared to those in both the control and LPS-activated BMs (panel B in Fig. 4). These results show that dapsone can induce cell death in BMs via disturbance of the MMP.
Quantified ELISA specific for TNF-α was used to investigate whether dapsone may affect cytokine production of BMs treated with LPS. Despite the low concentration (25 µg/mL) treatment, dapsone significantly decreased the production of TNF-α in LPS-activated BMs by 54% (panel A in Fig. 5). This result indicates that dapsone can down-regulate the production of pro-inflammatory cytokines in BMs. We also investigated whether dapsone can modulate ROS in BMs; however, the results showed that dapsone did not change the level of ROS production in BMs (panel B in Fig. 5).
Anti-inflammatory drugs include steroid and non-steroidal anti-inflammatory drugs that can disturb cyclooxygenase [513]. Dapsone is commonly used as an antibiotic to treat leprosy [1617]. Though its anti-microbial mechanisms have been studied, the role of dapsone in inflammation is not clear yet. To elucidate the mechanism of dapsone on inflammatory cells, we cultured BMs in the presence of LPS to simulate an inflammatory environment. LPS is a representative inflammatory agent and causing endotoxemia, a kind of sepsis in hosts [15].
The effect of dapsone on the cellular activity and viability of LPS-activated BMs was measured by using MTT assays and nuclear/cellular staining with fluorescent dyes. Dapsone consistently damaged the viability of LPS-activated BMs. Flow cytometry analysis revealed that dapsone controls LPS-generated inflammatory cells, especially granulocytes. While the percentage of Gr-1 positive cells was 57% in control, LPS increased that level to 75%, and LPS plus dapsone treatment decreased the LPS level to 64%. These results suggest that dapsone has immunomodulatory effects through down-regulation of the excessive ratio of inflammation-related cells in BMs produced by LPS.
The present study demonstrated that dapsone can decrease the percentage of granulocytes in LPS-activated BMs. This result may be related to agranulocytosis, one of the reported side effects of dapsone [26]. However, our study was performed in BMs with an excessive inflammatory response generated by LPS, not under normal conditions. Thus, the effect is different from agranulocytosis. Flow cytometry analysis demonstrated that LPS enhanced the MMP of BMs, whereas dapsone profoundly decreased the LPS-increased MMP of BMs. The observation that dapsone can inhibit the MMP in inflammatory cells or granulocytes has not been previously reported. The present results show that dapsone reduces both number and mitochondrial function of inflammatory cells when LPS is present.
To investigate further the anti-inflammatory effects of dapsone, we measured the production of TNF-α in LPS-activated BMs. TNF-α is a representative pro-inflammatory cytokine and is essentially produced during a variety of inflammatory reactions [411]. Interestingly, dapsone at a low concentration (25 µg/mL) significantly decreased the production of TNF-α by 54% from that in LPS-treated BMs. This result may broaden the uses of dapsone to include down-regulating the production of inflammatory cytokines.
Taken together, this study has demonstrated that dapsone can modulate the abundance of inflammatory cells, including granulocytes, in LPS-activated BMs by inducing cell death and down-regulating TNF-α production. Considering that dapsone is widely used as an antibiotic, and its safety is secured, our results indicate the possibility of developing it as an anti-inflammatory drug.
Figures and Tables
Fig. 1
Dapsone decreases the cellular activity of bone marrow cells (BMs) activated by lipopolysaccharide (LPS). BMs (1 × 106 cells/mL) were incubated in 96-well culture plates and treated with dapsone at the indicated concentrations (0–100 µg/mL) in the absence or presence of 1 µg/mL LPS. After 3 days, MTT assays were performed. Data are presented as mean ± SD values. ***p < 0.001, compared to BMs treated with LPS alone (dapsone 0 µg/mL). ###p < 0.001, between BMs treated with LPS and no LPS in the same concentration of dapsone. OD, optical density.

Fig. 2
Dapsone decreases the lipopolysaccharide (LPS)-produced increase in the percentage of granulocytes. Bone marrow cells (BMs; 1 × 106 cells/mL) were seeded in 6-well culture plates and treated with 1 µg/mL LPS and 50 µg/mL dapsone separately or together. (A) Dot plot of cell sizes (forward scatter/side scatter [FSC/SSC]). (B) Relationship between granularity and Gr-1 expression (Gr-1/SSC) in BMs was measured by flow cytometry.

Fig. 3
Dapsone affects the morphological changes of lipopolysaccharide (LPS)-treated bone marrow cells. The cells were cultured and treated as described in Fig. 2. Photograph images were obtained using an inverted microscope (100×) and a digital camera.
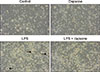
Fig. 4
Dapsone diminishes the mitochondrial membrane potential (MMP) and increases cell death in lipopolysaccharide (LPS)-activated bone marrow cells (BMs). BMs were cultured and treated as described in Fig. 2. To measure the MMP (A) and cell death (B), the treated BMs were stained with rhodamine 123 and propidium iodide (PI) solutions, respectively. The number in the histograms represents geometric mean fluorescence intensity (A) and the percentage in the dot plots indicates the portion of dead cells in the rectangular (low forward scatter [FSC]/high PI) region (B).
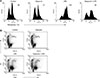
Fig. 5
Effects of dapsone on the production of tumor necrosis factor alpha (TNF-α) and reactive oxygen species (ROS) in lipopolysaccharide (LPS)-activated bone marrow cells (BMs). (A) BMs were cultured at a concentration of 1 × 106 cells/mL in 96-well culture plates and treated with dapsone (0–100 µg/mL) and/or LPS (1 µg/mL). After 3 days, the culture supernatants were harvested and used for enzyme-linked immunosorbent assay. Data are presented as mean ± SD values. *p < 0.05 and **p < 0.01, respectively, compared to BMs treated with LPS alone (dapsone 0 g/mL). ND, none detectable. (B) BMs were cultured and treated as described in Fig. 2. The cells were harvested after 3 days and stained with dichlorofluorescein diacetate. The numbers in the histograms indicate the geometric mean fluorescence intensity.

Acknowledgments
This work was supported by Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry (IPET) through Agri-Bio Industry Technology Development Program funded by Ministry of Agriculture, Food and Rural Affairs (MAFRA) (grant No. 111065-3).
References
1. Abe M, Shimizu A, Yokoyama Y, Takeuchi Y, Ishikawa O. A possible inhibitory action of diaminodiphenyl sulfone on tumour necrosis factor-α production from activated mononuclear cells on cutaneous lupus erythematosus. Clin Exp Dermatol. 2008; 33:759–763.

2. Andersohn F, Konzen C, Garbe E. Systematic review: agranulocytosis induced by nonchemotherapy drugs. Ann Intern Med. 2007; 146:657–665.

3. Aratani Y. Myeloperoxidase: its role for host defense, inflammation, and neutrophil function. Arch Biochem Biophys. 2018; 640:47–52.

4. Carswell EA, Old LJ, Kassel RL, Green S, Fiore N, Williamson B. An endotoxin-induced serum factor that causes necrosis of tumors. Proc Natl Acad Sci U S A. 1975; 72:3666–3670.

5. Chan CC, Rodger IW. Selective cyclooxygenase-2 inhibitors as potential therapeutic agents for inflammatory diseases. Adv Exp Med Biol. 1997; 407:157–161.

6. Coleman MD. Dapsone-mediated agranulocytosis: risks, possible mechanisms and prevention. Toxicology. 2001; 162:53–60.

7. Jang JY, Moon SY, Joo HG. Differential effects of fucoidans with low and high molecular weight on the viability and function of spleen cells. Food Chem Toxicol. 2014; 68:234–238.

8. Kanoh S, Tanabe T, Rubin BK. Dapsone inhibits IL-8 secretion from human bronchial epithelial cells stimulated with lipopolysaccharide and resolves airway inflammation in the ferret. Chest. 2011; 140:980–990.

9. Kettle AJ, Winterbourn CC. Mechanism of inhibition of myeloperoxidase by anti-inflammatory drugs. Biochem Pharmacol. 1991; 41:1485–1492.

10. Kim HJ, Kim MH, Byon YY, Park JW, Jee Y, Joo HG. Radioprotective effects of an acidic polysaccharide of Panax ginseng on bone marrow cells. J Vet Sci. 2007; 8:39–44.

11. Matsukawa A, Yoshinaga M. Sequential generation of cytokines during the initiative phase of inflammation, with reference to neutrophils. Inflamm Res. 1998; 47:Suppl 3. S137–S144.

12. Perera PY, Mayadas TN, Takeuchi O, Akira S, Zaks-Zilberman M, Goyert SM, Vogel SN. CD11b/CD18 acts in concert with CD14 and Toll-like receptor (TLR) 4 to elicit full lipopolysaccharide and taxol-inducible gene expression. J Immunol. 2001; 166:574–581.

13. Polisson R. Nonsteroidal anti-inflammatory drugs: practical and theoretical considerations in their selection. Am J Med. 1996; 100:31S–36S.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download