Abstract
Background
Methods
Results
ACKNOWLEDGMENTS
References
SUPPLEMENTARY MATERIAL
Supplementary Table 1
Table 1
Clinical and laboratory characteristics according to education in men
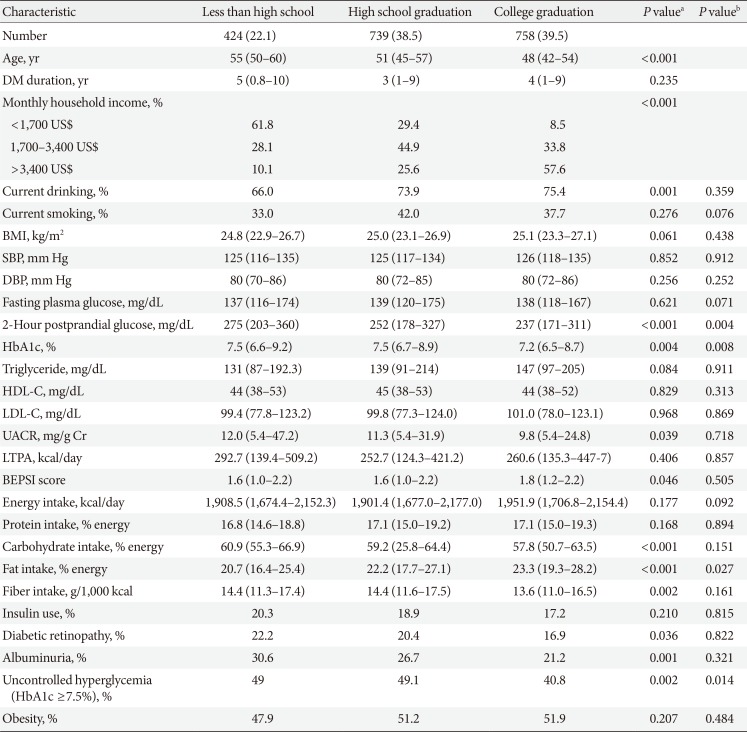
Values are presented as number (%) or median (interquartile range).
DM, diabetes mellitus; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; HbA1c, glycosylated hemoglobin; HDL-C, high density lipoprotein cholesterol; LDL-C, low density lipoprotein cholesterol; UACR, urinary albumin creatinine ratio; LTPA, leisure time physical activity; BEPSI, Brief Encounter Psychosocial Instrument.
aP values were calculated by Spearman's correlation for continuous variables and Mantel-Haenszel test of trend for categorical variables, bPartial Spearman's correlation and logistic regression was used to calculate P values after adjustment for age, diabetes duration, and income.
Table 2
Clinical and laboratory characteristics according to monthly household income in men
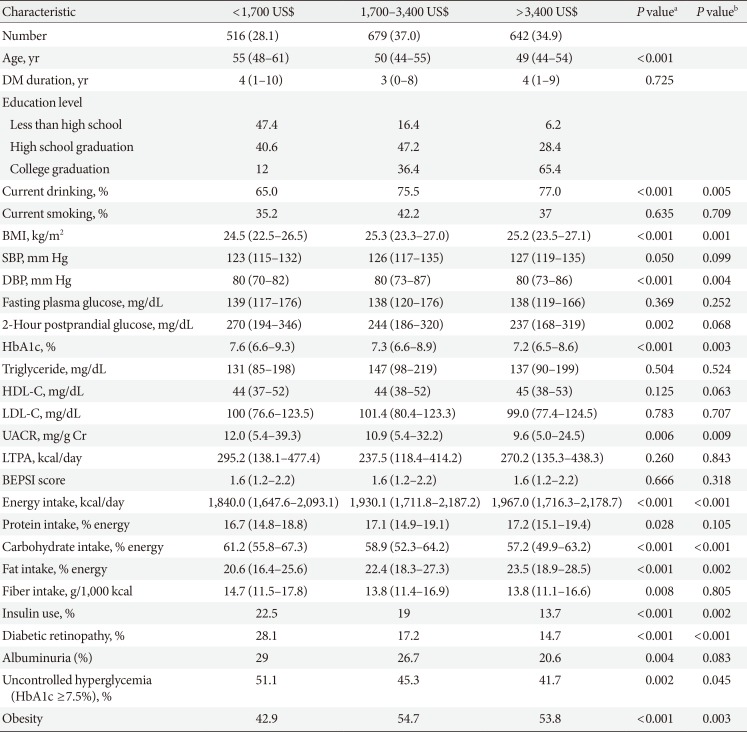
Values are presented as number (%) or median (interquartile range).
DM, diabetes mellitus; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; HbA1c, glycosylated hemoglobin; HDL-C, high density lipoprotein cholesterol; LDL-C, low density lipoprotein cholesterol; UACR, urinary albumin creatinine ratio; LTPA, leisure time physical activity; BEPSI, Brief Encounter Psychosocial Instrument.
aP values were calculated by Spearman's correlation for continuous variables and Mantel-Haenszel test of trend for categorical variables, bPartial Spearman's correlation and logistic regression was used to calculate P values after adjustment for age, diabetes duration, and education.
Table 3
Clinical and laboratory characteristics according to education in women
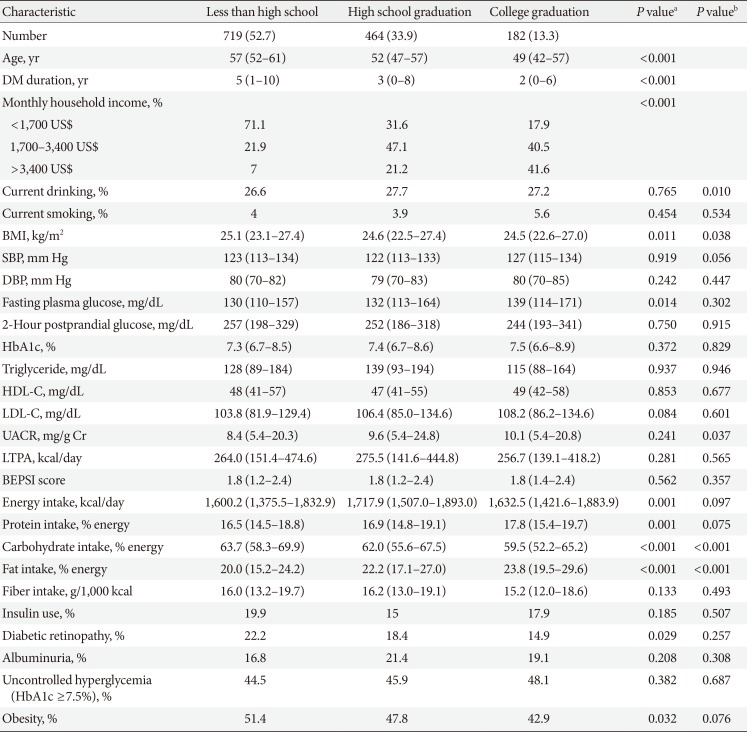
Values are presented as number (%) or median (interquartile range).
DM, diabetes mellitus; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; HbA1c, glycosylated hemoglobin; HDL-C, high density lipoprotein cholesterol; LDL-C, low density lipoprotein cholesterol; UACR, urinary albumin creatinine ratio; LTPA, leisure time physical activity; BEPSI, Brief Encounter Psychosocial Instrument.
aP values were calculated by Spearman's correlation for continuous variables and Mantel-Haenszel test of trend for categorical variables, bPartial Spearman's correlation and logistic regression was used to calculate P values after adjustment for age, diabetes duration, and income.
Table 4
Clinical and laboratory characteristics according to monthly household income in women
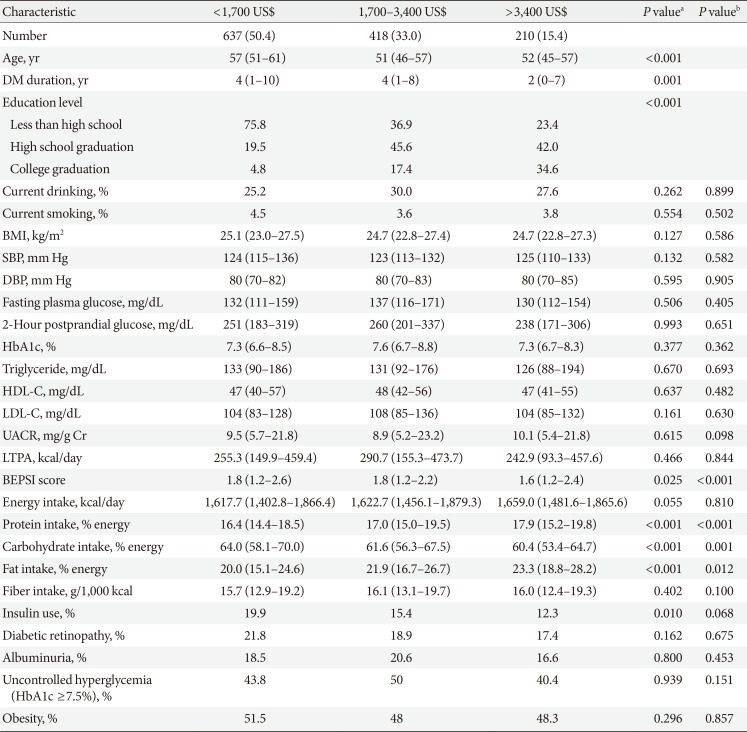
Values are presented as number (%) or median (interquartile range).
DM, diabetes mellitus; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; HbA1c, glycosylated hemoglobin; HDL-C, high density lipoprotein cholesterol; LDL-C, low density lipoprotein cholesterol; UACR, urinary albumin creatinine ratio; LTPA, leisure time physical activity; BEPSI, Brief Encounter Psychosocial Instrument.
aP values were calculated by Spearman's correlation for continuous variables and Mantel-Haenszel test of trend for categorical variables, bPartial Spearman's correlation and logistic regression was used to calculate P values after adjustment for age, diabetes duration, and education.
Table 5
Odds ratios of education and income levels on uncontrolled hyperglycemia and obesity by gender
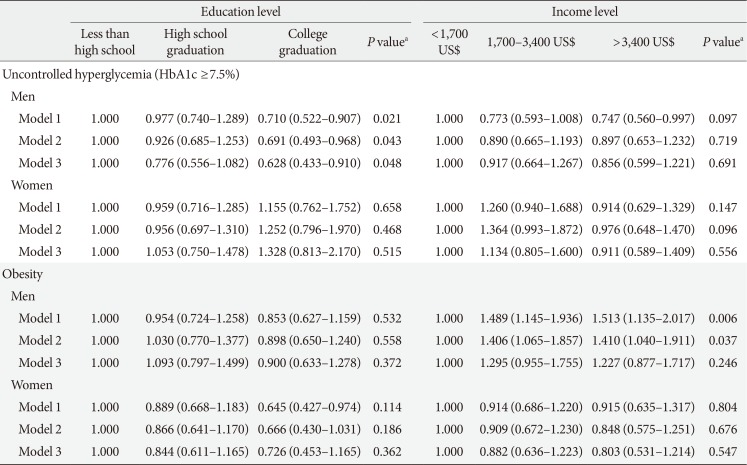
Values are presented as adjusted odds ratios (95% confidence interval) for logistic regression. Model 1: adjustment for age, duration, smoking, drinking, and education or income level; Model 2: adjustment for age, duration, smoking, drinking, education or income level, and insulin use; Model 3: adjustment for age, duration, smoking, drinking, education or income level, insulin use, energy intake, and carbohydrate and fat intake.
HbA1c, glycosylated hemoglobin.
aP values were calculated by the trend test.
Table 6
Odd ratios of education and income levels on diabetic retinopathy by gender
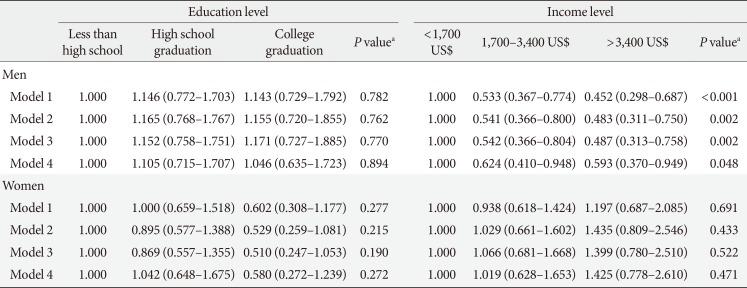
Values are presented as adjusted odds ratios (95% confidence interval) for logistic regression. Model 1: adjustment for age, duration, education or income level, smoking, and drinking; Model 2: adjustment for age, duration, education or income level, current smoking, current drinking, and insulin use; Model 3: adjustment for age, duration, education or income level, smoking, drinking, insulin use, glycosylated hemoglobin (HbA1c), systolic blood pressure (BP), and diastolic BP; Model 4: adjustment for age, duration, education or income level, smoking, drinking, insulin use, HbA1c, systolic BP, diastolic BP, energy intake, and carbohydrate and fat intake.
aP values were calculated by the trend test.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download