Abstract
Lymphomatoid granulomatosis (LYG) is an angiocentric and angiodestructive neoplastic proliferation of B and T lymphocytes commonly involving the lungs. Epstein-Barr virus is commonly detected in lesional cells. We report a case of a 54-year-old female with underlying monoclonal gammopathy of unknown significance who presented with a 4 week history of dyspnea and cough. Computed tomography scan of the chest showed multiple lung nodules as well as endobronchial narrowing causing atelectasis at the left upper lobe. Bronchoscopic findings revealed obstruction at the lingula segment due to endobronchial mass as a rare presentation. Bronchoscopic biopsy was diagnosed with LYG grade 1. After treatment, the endobronchial mass and lung lesions were completely resolved. However, the patient eventually evolved to malignant lymphoma after 1 year.
Lymphomatoid granulomatosis (LYG) is a rare Epstein-Barr virus (EBV) driven B-cell lymphoproliferative disorder. Atypical lymphoid cells directly accumulate within the affected tissues and clinically present in the form of infiltrative nodular lesions and with T-cell invasion and blood vessel destruction1. LYG most frequently involve the lung, but other common sites of involvement are the brain, kidney, liver, and skin2. Most present as multiple lung nodules, predominantly involving the lung bases, but occasionally atypical radiologic features can appear.
Here, we present a case of low grade pulmonary LYG with multiple lung lesions and endobronchial mass which is a rare presentation.
A 54-year-old female was admitted with a 1-month history of dyspnea, cough and pulmonary infiltrates. She was a never smoker and had no preexisting lung disease. She was diagnosed with monoclonal gammopathy of unknown significance (MGUS) and autoimmune hemolytic anemia 4 years ago, and treated with cyclophosphamide previously.
On admission, her initial vital signs were stable and physical examination was unremarkable. Her white blood cell count was 6,860/L, hemoglobin was 8.7 mg/dL, and platelet count was 148,000/mm3. In blood chemistry, high sensitivity C-reactive protein (hs-CRP) was 0.32 mg/dL, lactate dehydrogenase levels increased to 1,238 U/L, total bilirubin was 1.90 mg/dL, and direct bilirubin was 0.53 mg/dL. During admission, the patient developed fever up to 39.7℃ with respiratory symptoms and hs-CRP increased to 2.06 mg/dL.
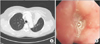
Initial chest radiograph showed diffuse patchy and nodular densities in the left lower lung (Figure 1A) and a low dose computed tomography of chest revealed variable sized round nodules and segmental collapse with proximal endobronchial filling defect in the left upper lingula segment (Figure 1B, C).
Bronchoscopic examination revealed diffuse bronchial nodular lesions with hypervascular mucosal changes and luminal narrowing at both bronchi (Figure 2A). Especially, left upper lobe (LUL) lingula division was almost nearly obstructed due to nodular lesion (Figure 2B). Endobronchial biopsy was performed at lingula division. Histopathologic examination showed infiltration with polymorphic atypical lymphoid cells and histiocytes (Figure 3A). Immunohistochemistry for CD3, CD20 as well as in situ hybridization for EBV were performed. Special stains demonstrated that the atypical population was characterized by many CD3-positive small reactive T cells (Figure 3B) with some scattered large CD20-positive B cells (Figure 3C). In situ hybridization for EBV-encoded RNA revealed a consistent number of EBV-positive large B cells (Figure 3D). This was consistent with a diagnosis of LYG grade 1.
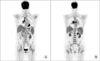
After corticosteroid pulse therapy, the patient's symptoms recovered and there was gradual but slow resolution of radiograph findings. After discharge, she was on maintenance corticosteroids. Nevertheless, after 8 months her respiratory symptoms recurred and computed tomography (CT) findings revealed LUL total collapse and follow-up bronchoscopy revealed total obstruction of LUL lingula segment (Figure 4). Cyclophosphamide therapy was started and 1 year later, there was complete regression of endobronchial tumor and atelectasis in LUL as well as multiple lung nodules (Figure 5). In spite of improvement in the lungs, 1 month later on, she developed enlargement of multiple lymph nodes in the neck, mediastinum and abdomen. Neck lymph node excisional biopsy was done and the result confirmed diffuse large B-cell lymphoma. Positron emission tomography CT results showed fludeoxyglucose uptakes in bilateral lower neck, mediastinum, peribronchial, mesentery and retroperitoneum nodes (Figure 6). The patient is receiving chemotherapy (R-CHOP) and is showing partial regression.
LYG is a rare, EBV related lymphoproliferative disorder that is classified by the number of EBV-positive atypical B cells3. Age of onset is ~30-50 years and male seems to be more susceptible than females2. LYG is seen in various immunodeficiency states, such as acquired immune deficiency syndrome, various autoimmune diseases, post-transplantation immunodeficiency, and use of immunosuppressant medications1,4.
Nearly 90% of patients are symptomatic at diagnosis and present with a 4- to 8-month history of general and respiratory symptoms. Respiratory symptoms, mainly cough and dyspnea are found in more than half of cases. Other symptoms such as weight loss, sweating, acute respiratory distress may be present1,2,5. Extrapulmonary site of involvement mainly affect the skin, nervous system, and kidneys1.
The most common radiographic feature of LYG is multiple bilateral lung nodules of variable size involving mainly the lung base1. The lesions can progress rapidly, coalesce and commonly cavitate, therefore mimicking Wegner's granulomatosis or metastases6. In rare cases, it can present as a solitary large pleural based mass, idiopathic interstitial pneumonia or a lung abscess6,7. In our case, along with multiple lung nodules, there was also an endobronchial mass causing atelectasis, which is a rare presentation. There have only been two reports that describe of endobronchial involvement in previous literature7. However, our case is the only case to show complete regression of endobronchial mass and atelectasis after therapy. The endobronchial involvement was confirmed with bronchoscopic biopsy.
Histopathologic finding of LYG is an angiocentric polymorphous mononuclear infiltrate composed of numerous small lymphocytes, plasma cells, histiocytes and large EBV-postive B cells, which resemble immunoblasts. The small lymphocytes are mainly T cells, which are negative for EBV8. Based on the number of EBV-positive large B cells, LYG can be divided into 3 grades. Grade 1 contains only a few EBV-positive B cells, and grade 3 contains almost entirely of EBV-positive large B cells, and is regarded as an aggressive lymphoma9.
There has been no standard treatment established and the outcome of this disease is poor. In addition, patients often respond initially, but relapse is very common8. Patients with lower-grade lesions may be observed for regression, but patients with high-grade LYG require immediate treatment2. Therapies include corticosteroids, anti-CD20 monoclonal antibodies such as rituximab, interferon-alpha-2b and combination chemotherapy including cyclophosphamide. Corticosteroids are the most common treatment of LYG, but relapse is the rule and they are not useful for long-term disease control1.
The prognosis of LYG is variable but generally poor. Twenty percent of stage 1 patients can achieve spontaneous remission, but the disorder is often progressive10. Studies have shown a median survival of 14 months and a mortality of 65%-90%7. Many patients die as a result of pulmonary hemorrhage, central nervous system involvement, or transformation to an aggressive lymphoma. Our case was interesting as the lung lesions of stage 1 LYG that showed complete remission after therapy, still transformed to an aggressive lymphoma later on.
There have only been three cases of pulmonary lymphomatoid granulomatosis reported in Korea11,12,13. This is the first report of low grade LYG developed from a patient with MGUS receiving cyclophosphamide treatment. In our case there was an endobronchial mass as well as lung nodules, which is an unusual manifestation. Our case suggests that bronchoscopic examinations are needed to confirm endobronchial involvements in LYG. We also emphasize that in low grade LYG, where disease regression is noted after therapy, there is always the possibility to transform into malignant lymphoma. This should be considered when treating low grade LYG.
Figures and Tables
Figure 1
(A) Initial chest posteroanterior image revealing diffuse patchy and nodular densities in the left lower lung. (B, C) Low dose computed tomography scan of chest on admission showing diffused patchy and nodular densities in both lungs and segmental collapse with proximal endobronchial filling defect in left upper lingula segment.

Figure 2
Bronchoscopy showing diffused bronchial nodular lesions with hypervascular mucosal changes and luminal narrowing at right upper lobe (A) and left upper lobe lingular division revealing almost near obstruction (B).
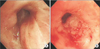
Figure 3
Pathology of endobronchial biopsy showing infiltration of lymphoid cells, scattered plasma cells, and histiocytes (A) at immunohistochemistry, a background of CD4+ T-cells (B) with CD20+ (C), and large atypical B cells with nuclear positivity for Epstein-Barr virus (EBV)-RNA (D) with EBV-encoded RNA probe after in-situ hybridization.
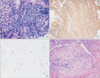
Figure 4
After 8 months of lymphomatoid granulomatosis diagnosis, computed tomography scan of chest showing left upper lobe (LUL) lobar bronchus obstruction and LUL total collapse (A) with bronchoscopy showing total obstruction of LUL bronchus by mass (B).
References
1. Roschewski M, Wilson WH. Lymphomatoid granulomatosis. Cancer J. 2012; 18:469–474.
2. Cadranel J, Wislez M, Antoine M. Primary pulmonary lymphoma. Eur Respir J. 2002; 20:750–762.
3. Wilson WH, Kingma DW, Raffeld M, Wittes RE, Jaffe ES. Association of lymphomatoid granulomatosis with Epstein-Barr viral infection of B lymphocytes and response to interferonalpha 2b. Blood. 1996; 87:4531–4537.
4. Fassas A, Jagannath S, Desikan KR, Shah HR, Shaver R, Waldron J, et al. Lymphomatoid granulomatosis following autologous stem cell transplantation. Bone Marrow Transplant. 1999; 23:79–81.
5. Katzenstein AL, Doxtader E, Narendra S. Lymphomatoid granulomatosis: insights gained over 4 decades. Am J Surg Pathol. 2010; 34:e35–e48.
6. Hare SS, Souza CA, Bain G, Seely JM, Frcpc , Gomes MM, et al. The radiological spectrum of pulmonary lymphoproliferative disease. Br J Radiol. 2012; 85:848–864.
7. Mohyuddin GR, Sultan F, Khaleeq G. A rare presentation of a rare disease: pulmonary lymphomatoid granulomatosis. Case Rep Pulmonol. 2012; 2012:371490.
8. Tagliavini E, Rossi G, Valli R, Zanelli M, Cadioli A, Mengoli MC, et al. Lymphomatoid granulomatosis: a practical review for pathologists dealing with this rare pulmonary lymphoproliferative process. Pathologica. 2013; 105:111–116.
9. Campo E, Swerdlow SH, Harris NL, Pileri S, Stein H, Jaffe ES. The 2008 WHO classification of lymphoid neoplasms and beyond: evolving concepts and practical applications. Blood. 2011; 117:5019–5032.
10. Jaffe ES, Wilson WH. Lymphomatoid granulomatosis: pathogenesis, pathology and clinical implications. Cancer Surv. 1997; 30:233–248.
11. Kwon OJ, Han SK, Shim YS, Kim KY, Han YC, Kim JH, et al. Lymphomatoid granulomatosis: a case report with review of literature. Korean J Intern Med. 1985; 29:124–130.
12. Jung HK, Cheon SH, Lee SN, Kim SS. A case of pulomonary lymphomatold granulomatosis. Korean J Med. 1997; 52:247–252.
13. Kim EH, Jang HJ, Rhee KH, Han EM, Hugh J, Kim SD, et al. A case of lymphomatoid granulomatosis refractory to various antilymphoma treatment including high dose therapy. Korean J Med. 2007; 72:S332–S337.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download