Abstract
Objective
To evaluate the prevalence of incidentally found unruptured intracranial aneurysms (UIAs) on the brain MR angiography (MRA) from a community-based general hospital.
Materials and Methods
This was a prospectively collected retrospective study, carried out from January 2004 to December 2004. The subjects included 3049 persons from a community-based hospital in whom MRA was performed according to a standardized protocol in an outpatient setting. Age- and sex-specific prevalence of UIAs was calculated. The results by MRA were compared with intra-arterial digital subtraction angiography (DSA) findings.
Results
Unruptured intracranial aneurysms were found in 137 (5%) of the 3049 patients (M:F = 43:94; mean age, 60.2 years). The prevalence of UIAs was 5% (n = 94) in women and 4% (n = 43) in men, respectively (p = 0.2046) and showed no age-related increase. The most common site of aneurysm was at the distal internal carotid artery (n = 64, 39%), followed by the middle cerebral artery (n = 40, 24%). In total, 99% of aneurysms measured less than 12 mm, and 93% of aneurysms measured less than 7 mm. Direct comparisons between MRA and DSA were available in 70 patients with 83 UIAs; the results revealed two false positive and two false negative results.
The estimates for the prevalence of unruptured intracranial aneurysms (UIAs) vary between 0.2 and 9% of the population, depending on angiographic and autopsy studies (1-6). In subsequent larger studies (7-9), UIAs were detected in 2% to 3% of healthy subjects who underwent MR angiography (MRA). This wide range may reflect methodological differences between studies. Precise prevalence of UIAs is mandatory for the evaluation of testing results for the screening programs for aneurysms in the general population, and the development of a strategy for the management for UIAs, which are influenced by prevalence.
Although intra-arterial digital subtraction angiography (DSA) remains the gold standard for aneurysm delineation, many reports have described the use of MRA as equivalent to DSA for the detection of the vast majority of UIAs because of improved capability of MRI for detecting subtle or small brain abnormalities by higher resolution, field strength, and with more sensitive sequences (10-12).
Thus, this report describes a community-based general hospital study that was conducted to estimate the prevalence of incidentally found UIA on the basis of MRA.
This retrospective study of prospectively collected samples was conducted in a community-based general hospital in suburban Seoul, Korea. From January 2004 to December 2004, a total of 3049 consecutive patients (M:F = 1118:1931; mean age, 56.3 years; age range, 15-88) underwent MRA for various causes including a routine health check-up at an outpatient clinic. Patients were eligible for enrollment if they had incidental (found by chance) or symptomatic, but UIA. Patients were excluded if they had any sign of: 1) ruptured aneurysms (n = 9); 2) previously clipped aneurysms (n = 21); 3) fusiform, traumatic, and mycotic aneurysms (n = 2); or 4) extradural aneurysms (n = 5). Patients who were thought to be possible candidates for surgical treatment were advised to undergo DSA.
All MRI examinations were performed on a 1.5-Tesla (T) system with a standard head coil (Philips Medical Systems, Best, The Netherlands). The three dimensional (3D) time-of-flight (TOF) MRA technique was used with the following parameters: Multiple overlapping thin slabs; TR/TE, 27/6.9; flip angle, 20°; three 48 mm slabs; 48 partitions for each slab; effective section thickness, 0.8 mm; matrix, 512 × 256; field of view, 20 cm; one excitation; acquisition time, and less than 10 min. The source images were transferred to a workstation (Philips Medical Systems) running the Advantage Windows software (Easy Vision®; Philips Medical Systems). Maximum intensity projection (MIP), and volume rendering images were created for each data set to evaluate the intracranial aneurysms. Initially, in each of the MIP images, anterior and posterior circulation was interactively rotated around axial and sagittal axes at 15° increments to examine the presence of harbored aneurysms. Further arbitrary oblique projections were also obtained when necessary, to overcome vascular overlapping. As a second step, each detected aneurysm on the MIP images was separately evaluated using targeted volume rendering images to examine the aneurysm morphology and size.
Intra-arterial DSA studies were performed on a digital angiography system (LC plus; GE, Milwaukee, WI) with a 512 × 512 pixel matrix. Three- or four-vessel angiography were used, including both internal carotid arteries (ICAs) and vertebral arteries. With each injection, standard anteroposterior and lateral projections were routinely acquired, with additional views (oblique, transfacial, and contralateral carotid compression) obtained when necessary.
We calculated the prevalence of UIAs in terms of age distribution and gender difference. For comparison of the incidence of aneurysms, the 3049 patients were classified according to the following age groups: less than 20, 20 to 39, 40 to 59, 60 to 79, and higher than 80 years.
All MR images were retrospectively and independently reviewed by two dedicated neuroradiologists, one of whom has been practicing in the field of neurointervention. They were unaware of the clinical information and DSA findings. In cases of discordant results, a consensus interpretation by the two readers was completed. The readings were performed with a digital picture archiving and communication system (PACS) (PathSpeed, or Centricity 2.0; GE Healthcare, Mt. Prospect, IL), using MIP images first, and then, a thorough review of source images, and volume rendering projections. The MR images were analyzed, with particular attention to the aneurysm number, location, and size. The name used to describe the aneurysm was based on the junction of the secondary artery arising from the parent artery. The aneurysms were independently measured on a workstation and it was determined in the transverse plane on the axial source images to be the largest diagonal measurement, including any appendages. The superior to inferior dimension was also measured; the final aneurysm size was defined as the larger of the transverse or superior to inferior measurements.
Correlations with cerebral four vessels DSA were available in 70 patients with 83 UIAs. DSA was performed within 90 weeks (range, 1-90 weeks; mean, 12.5 weeks). We classified these 83 aneurysms on the MRA as definite and suspicious groups. A definite aneurysm on MRA was defined when there was a clear demarcation between the parent artery and neck portion of the aneurysm on MRA including source, MIP, and volume rendering images. A suspicious aneurysm on MRA was defined when there was no clear demarcation between the parent artery and the neck portion of the aneurysm. However, there are differences in vessel diameter which were depicted by focal widening or ecstatic change. Then, the results from DSA were compared with the findings from MRA to assess the accuracy of MRA in the diagnosis of UIAs. DSA data were reviewed independently by an interventional neuroradiologist. For cases in which DSA findings differed from MRA findings, the DSA findings were considered as a standard of reference.
Fisher's exact test was used to compare the proportion of UIAs according to gender.
Of the 3049 patients examined, 164 UIAs were found in 137 patients (M:F = 43:94; mean age, 60.2 years; age range, 20-87 years). The age- and sex-specific prevalence of UIAs was shown in Table 1. UIAs were found in 137 (of 3049 patients, 5%) patients, including 43 men (of 1118, 4%) and 94 women (of 1931, 5%). The patients presented as follows: 1) neurologic symptoms (n = 107); headache (n = 51); ischemic cerebrovascular disease (n = 31); dizziness (n = 22), and cranial nerve deficit (n = 3), 2) nonspecific symptoms without neurologic findings (n = 5), 3) for a routine health check-up (n = 25). Twenty-one patients (15%) had multiple aneurysms and in all, a total of 164 aneurysms were detected. No aneurysms were found in 133 young patients aged under 20 years and the prevalence of UIAs showed no relationship with age. One peak appeared in the 60 to 79 years age group (6%), both in male (5%) and female (6%) patients. The prevalence of UIAs was higher in women (5%, 94 of 1931) than in men (4%, 43 of 1118), but the difference was not statistically significant (p = 0.2046).
The distribution of aneurysm locations and sizes for patients who underwent MRA were summarized in Table 2. The most frequent site was the distal internal carotid artery where 64 aneurysms (39%) occurred. In particular, 56 (34%) occurred at the paraclinoid portion of the internal carotid artery, followed by 40 (24%) at the middle cerebral artery, 22 (13%) at the posterior communicating artery, 20 (12%) at the anterior communicating artery, 12 (7%) at the vertebrobasilar artery, and six (4%) at the anterior cerebral artery. The diameters of the aneurysms ranged from 2 to 20 mm. In 99% of aneurysms, they measured less than 12 mm. Moreover, 93% of aneurysms were less than 7 mm, and 15% of aneurysms were less than 3 mm.
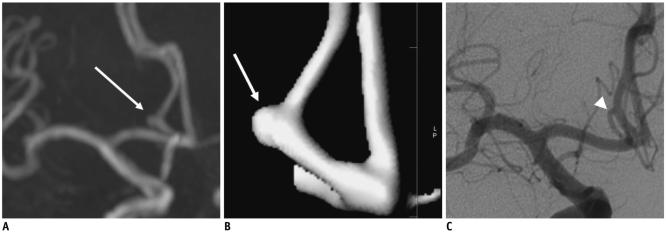
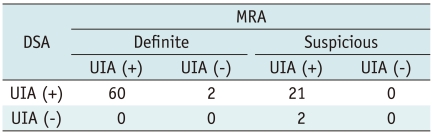
MR angiography results were compared with DSA findings in 83 UIAs of 70 patients on Table 3. In four (4 of 83, 5%) cases, the DSA revealed different findings compared with MRA. Specifically, two cases of a MRA-positive aneurysm found that the DSA findings were completely normal, showing no aneurysms; whereas, DSA showed two additional aneurysms which were not identified by MRA. The false negative aneurysms measured 2 mm and 3.5 mm, respectively and these were located in the middle cerebral artery and cavernous internal carotid artery (Fig. 1), while false positive aneurysms measured less than 3 mm in size and these were located in the anterior communicating artery and anterior cerebral artery on MRA (Fig. 2).
In the present study, the prevalence of UIAs on MRA (5%, 137 of 3049) was higher than previously reported (7-9). Horikoshi et al. (8) reported the UIAs prevalence rate of 3% in patients 40 years of age or older and Yue et al. (13) showed that UIAs were present in only 0.1% of patients 65 years of age or older. However, our result is in the range of prevalence of UIAs in autopsy studies. One plausible explanation is the state-of-the art scanning protocol, which incorporates more sensitive sequences such as MRA including source and volume rendering techniques. It might have yielded good detectability of smaller intracranial aneurysms. The TOF MRA, performed via a volume rendering technique, facilitates the visualization of the vascular surface and intravascular details, while preserving spatial relationships and improves the delineation of small caliber vessels (parent and branch vessels) and the perception of a lobulated aneurysm surface, which contributes to the precision in assessing aneurysm size (14). The other possible reason is the neurointerventionist's concern for detection of UIAs. In addition, selection bias may exist in our study because our study group included some symptomatic patients.
There has been controversy for the age-related increase in the prevalence of UIAs. Earlier studies (3, 8) have reported that the prevalence of UIAs steadily increased with age in both genders, whereas subsequent studies (6, 15) were similar to ours in showing that UIAs have no age-related increase in prevalence. This is likely attributed to the association with a small sample size of the over 80 year old age group. The female predominance of UIAs has been well demonstrated in autopsy or angiographic studies (6, 8). This sex-linked trend was found in our study as in other studies, although the difference was not statistically significant.
More than 90% of UIAs found using autopsy or angiography are less than 10 mm in diameter (3, 16). In our study, most of the aneurysms (93%) were smaller than 7 mm. Further, 20 patients (15%) had aneurysms less than 3 mm. Based on the recent International Study of Unruptured Intracranial Aneurysms (ISUIA), UIAs involving anterior circulation including anterior communicating artery, rupture rates were 0%, 3%, 15%, and 40%, respectively, for UIAs less than 7 mm, 7-12 mm, 13-24 mm, and 25 mm or greater. In comparison, rupture rates were 3%, 15%, 18%, and 50%, respectively, for the same size categories for UIAs involving posterior circulation including the posterior communicating artery (17). However, several experienced interventional neuroradiologists and neurosurgeons reported that, in practice, many of the ruptured aneurysms encountered were small (18-20). According to the recent reports, the prevalence of very small aneurysms (less than 3 mm) ranged from 7 to 15% of all ruptured aneurysms (20-22). Although no clear worldwide consensus exists on whether to treat patients with small UIAs, small UIAs may not be safe, as reported in the ISUIA study. Most of the aneurysms (93%) were located in the anterior circulation. UIAs apparently develop in different locations to ruptured aneurysms (3, 6, 23). Middle cerebral artery and anterior communicating artery aneurysms tended to be more common in previous studies (6), unlike our study, in which the most frequent locations of UIAs were was the paraclinoid portion of the internal carotid artery; this result was consistent with a previous study (24).
Similarly, another previous report (25) showed common findings with our study in the presence of false-positive lesions smaller than 3 mm in the anterior communicating artery region using MRA, and speculated that it was likely related to the association of loop formations, vessel overlaps, flow signal loss, atherosclerotic plaque, or turbulent flow.
A major strength of our study is the uniform MRI protocol for all subjects. The reviewers were unaware of the characteristics of the subjects, making detection bias unlikely. We used high-resolution, state of the art imaging sequences of MRI. MRA has been increasingly used in research and clinical medicine. Although DSA remains the gold standard in the diagnostic workup of intracranial aneurysms, it is not performed unless the patient is symptomatic or an additional imaging study has been suspicious for an aneurysm. MRI and MRA are less invasive, less expensive, and have lower risks than DSA. A potential limitation with respect to the generalizability of our study results is the fairly homogenous composition of a hospital-based and geographically defined study population. Moreover, our study population may not reflect the demographic population because it was based mainly on patients with clinical encounters. Another drawback is the lack of diagnostic standards for comparison with our consensus MRA findings. Our false-positive results might be higher when all of the patients underwent DSA.
In conclusion, this community-hospital based study suggests a higher prevalence of UIAs observed by MRA than previously reported. These findings should be anticipated for the incidental detection of UIAs in healthy subjects who undergo brain imaging as a part of a health checkup examination.
References
1. Stehbens WE. Aneurysms and anatomical variation of cerebral arteries. Arch Pathol. 1963; 75:45–64. PMID: 14087271.
2. Stehbens WE. Pathology of the cerebral blood vessels. 1972. St. Louis: C.V. Mosby.
3. Rinkel GJ, Djibuti M, Algra A, van Gijn J. Prevalence and risk of rupture of intracranial aneurysms: a systematic review. Stroke. 1998; 29:251–256. PMID: 9445359.
4. Housepian EM, Pool JL. A systematic analysis of intracranial aneurysms from the autopsy file of the Presbyterian Hospital, 1914 to 1956. J Neuropathol Exp Neurol. 1958; 17:409–423. PMID: 13564252.
5. Chason JL, Hindman WM. Berry aneurysms of the circle of Willis; results of a planned autopsy study. Neurology. 1958; 8:41–44. PMID: 13493689.

6. Iwamoto H, Kiyohara Y, Fujishima M, Kato I, Nakayama K, Sueishi K, et al. Prevalence of intracranial saccular aneurysms in a Japanese community based on a consecutive autopsy series during a 30-year observation period. The Hisayama study. Stroke. 1999; 30:1390–1395. PMID: 10390312.
7. Rocque BG, Baskaya MK, Kuo JS. Incidental findings on brain MRI. N Engl J Med. 2008; 358:853. author reply 854-855. PMID: 18287610.

8. Horikoshi T, Akiyama I, Yamagata Z, Nukui H. Retrospective analysis of the prevalence of asymptomatic cerebral aneurysm in 4518 patients undergoing magnetic resonance angiography--when does cerebral aneurysm develop? Neurol Med Chir (Tokyo). 2002; 42:105–112. discussion 113. PMID: 11936051.
9. Ross JS, Masaryk TJ, Modic MT, Ruggieri PM, Haacke EM, Selman WR. Intracranial aneurysms: evaluation by MR angiography. AJNR Am J Neuroradiol. 1990; 11:449–455. PMID: 2112306.

10. White PM, Wardlaw JM, Easton V. Can noninvasive imaging accurately depict intracranial aneurysms? A systematic review. Radiology. 2000; 217:361–370. PMID: 11058629.

11. Chung TS, Joo JY, Lee SK, Chien D, Laub G. Evaluation of cerebral aneurysms with high-resolution MR angiography using a section-interpolation technique: correlation with digital subtraction angiography. AJNR Am J Neuroradiol. 1999; 20:229–235. PMID: 10094343.
12. Grandin CB, Mathurin P, Duprez T, Stroobandt G, Hammer F, Goffette P, et al. Diagnosis of intracranial aneurysms: accuracy of MR angiography at 0.5 T. AJNR Am J Neuroradiol. 1998; 19:245–252. PMID: 9504473.
13. Yue NC, Longstreth WT Jr, Elster AD, Jungreis CA, O'Leary DH, Poirier VC. Clinically serious abnormalities found incidentally at MR imaging of the brain: data from the Cardiovascular Health Study. Radiology. 1997; 202:41–46. PMID: 8988190.

14. Mallouhi A, Felber S, Chemelli A, Dessl A, Auer A, Schocke M, et al. Detection and characterization of intracranial aneurysms with MR angiography: comparison of volume-rendering and maximum-intensity-projection algorithms. AJR Am J Roentgenol. 2003; 180:55–64. PMID: 12490476.

15. Vernooij MW, Ikram MA, Tanghe HL, Vincent AJ, Hofman A, Krestin GP, et al. Incidental findings on brain MRI in the general population. N Engl J Med. 2007; 357:1821–1828. PMID: 17978290.

16. Inagawa T, Hirano A. Autopsy study of unruptured incidental intracranial aneurysms. Surg Neurol. 1990; 34:361–365. PMID: 2244298.

17. Wiebers DO, Whisnant JP, Huston J 3rd, Meissner I, Brown RD Jr, Piepgras DG, et al. Unruptured intracranial aneurysms: natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet. 2003; 362:103–110. PMID: 12867109.

18. Guglielmi G, Vinuela F, Dion J, Duckwiler G. Electrothrombosis of saccular aneurysms via endovascular approach. Part 2: Preliminary clinical experience. J Neurosurg. 1991; 75:8–14. PMID: 2045924.
19. Russell SM, Lin K, Hahn SA, Jafar JJ. Smaller cerebral aneurysms producing more extensive subarachnoid hemorrhage following rupture: a radiological investigation and discussion of theoretical determinants. J Neurosurg. 2003; 99:248–253. PMID: 12924696.

20. Hwang JH, Roh HG, Chun YI, Kang HS, Choi JW, Moon WJ, et al. Endovascular coil embolization of very small intracranial aneurysms. Neuroradiology. 2011; 53:349–357. PMID: 20574735.

21. van Rooij WJ, Keeren GJ, Peluso JP, Sluzewski M. Clinical and angiographic results of coiling of 196 very small (< or = 3 mm) intracranial aneurysms. AJNR Am J Neuroradiol. 2009; 30:835–839. PMID: 19131407.
22. Gupta V, Chugh M, Jha AN, Walia BS, Vaishya S. Coil embolization of very small (2 mm or smaller) berry aneurysms: feasibility and technical issues. AJNR Am J Neuroradiol. 2009; 30:308–314. PMID: 19001535.

23. Inagawa T, Hada H, Katoh Y. Unruptured intracranial aneurysms in elderly patients. Surg Neurol. 1992; 38:364–370. PMID: 1485213.

24. Chae KS, Jeon P, Kim KH, Kim ST, Kim HJ, Byun HS. Endovascular coil embolization of very small intracranial aneurysms. Korean J Radiol. 2010; 11:536–541. PMID: 20808697.

25. Okahara M, Kiyosue H, Yamashita M, Nagatomi H, Hata H, Saginoya T, et al. Diagnostic accuracy of magnetic resonance angiography for cerebral aneurysms in correlation with 3D-digital subtraction angiographic images: a study of 133 aneurysms. Stroke. 2002; 33:1803–1808. PMID: 12105357.
Fig. 1
False-negative intracranial aneurysm on MR angiography in 58-year-old woman.
A, B. Maximum intensity projection image demonstrates two aneurysms (arrows) on right middle cerebral artery bifurcation (A) and M1 segment of left middle cerebral artery (B), respectively. C, D. Digital subtraction angiography image demonstrates another 2-mm-sized aneurysm (arrowhead) on M1 segment of left middle cerebral artery, in addition to two aneurysms seen on maximum intensity projection of MR angiography.
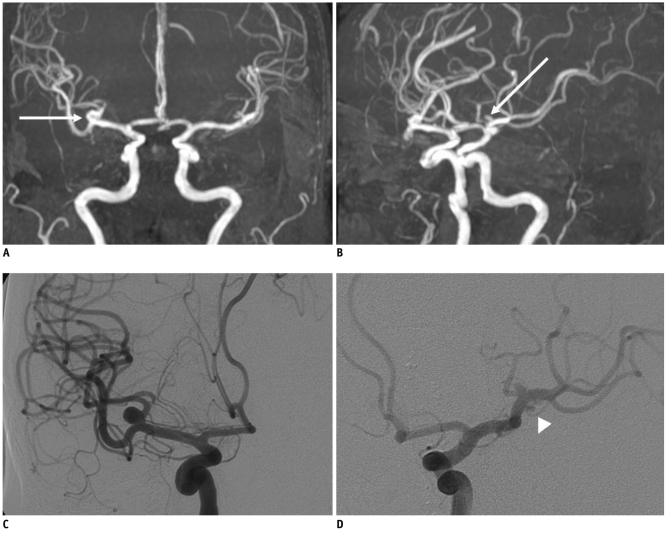
Fig. 2
False-positive intracranial aneurysm on MR angiography in 64-year-old woman.
A. Maximum intensity projection image demonstrates 2-mm-sized aneurysm (arrow) on A2 segment of left anterior cerebral artery. B. Volume rendering image shows presence of aneurysm (arrow) at same location. C. Digital subtraction angiography image demonstrates no aneurysm. Perforator (arrowhead) of anterior cerebral artery with downward direction mimics aneurysm.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download