INTRODUCTION
CASE DESCRIPTION
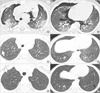
Case 1
Fig. 1
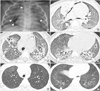
Case 2
Fig. 2
Journal List > J Korean Med Sci > v.33(16) > 1107778


Funding This study was funded by the Korea Ministry of Environment (MOE) as the Environmental Health Action Program (2016001360006).
Author Contributions
Conceptualization: Lee E, Do KH, Cho YA, Lee SY, Park DU, Hong SJ.
Data curation: Lee E, Son SK, Do KH, Cho YA, Hong SJ.
Validation: Lee E, Son SK, Do KH, Cho YA, Hong SJ.
Writing - original draft: Lee E, Do KH, Cho YA, Park DU, Hong SJ.
Writing - review & editing: Lee E, Do KH, Cho YA, Park DU, Hong SJ.
Eun Lee 
https://orcid.org/0000-0002-7462-0144
Seung Kook Son 
https://orcid.org/0000-0002-4902-5351
Jisun Yoon 
https://orcid.org/0000-0002-4904-9118
Hyun-Ju Cho 
https://orcid.org/0000-0003-4282-4000
Song-I Yang 
https://orcid.org/0000-0002-9648-4585
Sungsu Jung 
https://orcid.org/0000-0002-0559-4982
Kyung-Hyun Do 
https://orcid.org/0000-0003-1922-4680
Young Ah Cho 
https://orcid.org/0000-0002-3474-8653
So-Yeon Lee 
https://orcid.org/0000-0002-2499-0702
Dong-Uk Park 
https://orcid.org/0000-0003-3847-7392
Soo-Jong Hong 
https://orcid.org/0000-0003-1409-2113