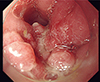
1. Williams GT, Bussey HJ, Morson BC. Inflammatory “cap” polyps of the large intestine. Br J Surg. 1985; 72:S133.
2. Mason M, Faizi SA, Fischer E, Rajput A. Inflammatory cap polyposis in a 42-year-old male. Int J Surg Case Rep. 2013; 4:351–353.
3. Li JH, Leong MY, Phua KB, Low Y, Kader A, Logarajah V, Ong LY, Chua JH, Ong C. Cap polyposis: a rare cause of rectal bleeding in children. World J Gastroenterol. 2013; 19:4185–4191.
4. Papaconstantinou I, Karakatsanis A, Benia X, Polymeneas G, Kostopoulou E. Solitary rectal cap polyp: case report and review of the literature. World J Gastrointest Surg. 2012; 4:157–162.
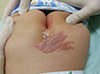
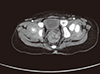
5. Mall V, Heinen F, Uhl M, Wellens E, Korinthenberg R. CNS lipoma in patients with epidermal nevus syndrome. Neuropediatrics. 2000; 31:175–179.
6. Géhénot M, Colombel JF, Wolschies E, Quandalle P, Gower P, Lecomte-Houcke M, Van Kruiningen H, Cortot A. Cap polyposis occurring in the postoperative course of pelvic surgery. Gut. 1994; 35:1670–1672.
7. Suzuki H, Sato M, Akutsu D, Sugiyama H, Sato T, Mizokami Y. A case of cap polyposis remission by betamethasone enema after antibiotics therapy including Helicobacter pylori eradication. J Gastrointestin Liver Dis. 2014; 23:203–206.
8. Oiya H, Okawa K, Aoki T, Nebiki H, Inoue T. Cap polyposis cured by Helicobacter pylori eradication therapy. J Gastroenterol. 2002; 37:463–466.
9. Akamatsu T, Nakamura N, Kawamura Y, Shinji A, Tateiwa N, Ochi Y, Katsuyama T, Kiyosawa K. Possible relationship between Helicobacter pylori infection and cap polyposis of the colon. Helicobacter. 2004; 9:651–656.
10. Abid S, Khawaja A, Bhimani SA, Ahmad Z, Hamid S, Jafri W. The clinical, endoscopic and histological spectrum of the solitary rectal ulcer syndrome: a single-center experience of 116 cases. BMC Gastroenterol. 2012; 12:72.
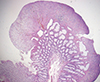
11. Singh B, Mortensen NJ, Warren BF. Histopathological mimicry in mucosal prolapse. Histopathology. 2007; 50:97–102.
12. Oriuchi T, Kinouchi Y, Kimura M, Hiwatashi N, Hayakawa T, Watanabe H, Yamada S, Nishihira T, Ohtsuki S, Toyota T. Successful treatment of cap polyposis by avoidance of intraluminal trauma: clues to pathogenesis. Am J Gastroenterol. 2000; 95:2095–2098.
13. Ng KH, Mathur P, Kumarasinghe MP, Eu KW, Seow-Choen F. Cap polyposis: further experience and review. Dis Colon Rectum. 2004; 47:1208–1215.
14. Bookman ID, Redston MS, Greenberg GR. Successful treatment of cap polyposis with infliximab. Gastroenterology. 2004; 126:1868–1871.
15. Maunoury V, Breisse M, Desreumaux P, Gambiez L, Colombel JF. Infliximab failure in cap polyposis. Gut. 2005; 54:313–314.
16. Isomoto H, Urata M, Nakagoe T, Sawai T, Nomoto T, Oda H, Nomura N, Takeshima F, Mizuta Y, Murase K, et al. Proximal extension of cap polyposis confirmed by colonoscopy. Gastrointest Endosc. 2001; 54:388–391.
17. Farajzadeh S, Zahedi MJ, Moghaddam SD. A new gastrointestinal finding in Proteus syndrome: report of a case of multiple colonic hemangiomas. Int J Dermatol. 2006; 45:135–138.
18. Romiti R, Arnone M, Sotto MN. Epidermal naevus with Darier’s disease-like changes in a patient with Gardner’s syndrome. J Am Acad Dermatol. 2000; 43:380–382.