Abstract
Figures and Tables
Fig. 1
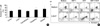
Fig. 2
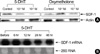
Fig. 3

Table 2

Bone marrow CD34+ cells (1×103) from normal donors were plated in 1 mL methylcellulose medium supplemented with 30% FBS and SCF (20 ng/mL), IL-3 (50 ng/mL), and EPO (6 U/mL). CFU-E and BFU-E (and CFU-GM) were enumerated on day-7 and day-14 of incubation, respectively. Hormones were added at the indicated concentrations when the cultures were started. The presented data are the means±SD of the number of colonies from triplicate cultures. The experiments were repeated three times, with similar results. Representative results are shown. *p<0.05 compared with untreated controls (containing 0.1% ethanol ).
Table 3

Bone marrow CD34+ cells from normal donors were incubated in serum-free medium in the presence or absence of hormones. After 36 hr, the cells were washed, and the number of cells equivalent to 1×103 input cells were plated in a methylcellulose clonogenic assay. CFU-E and BFU-E (and CFU-GM) were enumerated on day-7 and day-14 of incubation, respectively. The presented data are the means±SD of the number of colonies from triplicate cultures. The experiments were repeated three times, with similar results. Representative results are shown. *p<0.05 compared with untreated controls (containing 0.1% ethanol).
Table 4
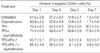
Bone marrow CD34+ cells from normal donors were incubated in IMDM for the indicated periods in the absence or presence of oxymetholone (1×10-5 M), alone or in combination with a mixture of cytokines (5 ng/mL TPO+5 ng/mL FL+5 ng/mL SCF; TFS) or IFN-γ (100 ng/mL), stained with annexin V and anti-CD34 antibody, and subjected to flow cytometric analysis. The presented data are the means±SD of annexin V-negative/CD34-positive cells from three independent experiments.
Table 5

Murine bone marrow stromal cells MS-5 were incubated in serum-free medium X-VIVO, with or without androgens at the indicated concentrations. After a 72-hr incubation, the concentrations of SDF-1α in the culture supernatants were measured using an ELISA. The presented data are the means±SD of three independent experiments. *p<0.05 compared with the untreated control (containing 0.1% ethanol) (analyzed by t-test for paired samples).
Table 6

Primary human bone marrow stromal cells were incubated in serum-free medium, with or without androgens at the indicated concentrations. After a 72-hr incubation, the concentrations of stem cell factor (SCF) in the culture supernatants were measured by ELISA. The presented data are the means±SD of triplicate experiments. *p<0.05 compared with the untreated untreated control (containing 0.1% ethanol) (analyzed by t-test for paired samples).
Table 7

Conditioned medium (CM) was prepared from primary human bone marrow stromal cells (BMSCs) in 25 culture flasks. When confluence was reached, the cultures were thoroughly rinsed, and 3 mL of serum-free X-VIVO medium was added to each flask, with or without the inclusion of hormones (10-5 M). After a 72-h incubation at 37℃, the supernatants were harvested. Normal BM CD34+ cells were incubated for 36 hr in CM, stained with annexin V and anti-CD34 antibody, and subjected to flow cytometric analysis. The presented data are the means±SD of annexin V-negative/CD34-positive cells from three independent experiments. *p<0.05 compared with the non-treated CM (analyzed by t-test for paired samples).




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download