Abstract
Purpose
To evaluate the therapeutic effects of topical heparin (Hylo-Parin®, Ursapharm Saarbr cken, Germany) and spray type phospholipids (Tears Again®, Optima Pharmazeutische GmbH. Freising, Germany) in severe dry eye syndrome resistant to conventional therapy.
Methods
Twenty eyes of ten patients with refractory severe dry eye were treated with Hylo-Parin® (two times a day) and Tears Again® (three times a day) for three months. Before and one and three months after treatment, a symptom questionnaire was administered to the patients. The ocular surface disease index (OSDI), tear film breakup time, Schirmer test, conjunctival fluorescein staining examinations and filamentary keratitis were evaluated.
Results
After using Tears Again® and Hylo-Parin®, the OSDI score improved from 64.13 ± 15.12 to 43.80 ± 15.87 (p<0.01). Tear film breakup time significantly increased from 1.0 ± 0.65 to 2.3 ± 0.73 seconds (p<0.01) and conjunctival staining score (Oxford scale) significantly decreased from 3.85 ± 0.75 to 3.25 ± 0.97 (p<0.01). Filamentary keratitis in the slit-lamp examination showed significant improvement (p<0.01).
References
1. The definition and classification of dry eye disease: report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop (2007). The Ocul Surf. 2007; 5:75–92.
2. Management and therapy of dry eye disease: report of the Management and Therapy Subcommittee of the International Dry Eye WorkShop (2007). The Ocular Surface. 2007; 5:163–78.
3. Bron AJ, Tiffany JM, Gouveia SM, et al. Functional aspects of the tear film lipid layer. Exp Eye Res. 2004; 78:347–60.

4. Albietz J, Sanfilippo P, Troutbeck R, Lenton LM. Management of filamentary keratitis associated with aqueous-deficient dry eye. Optom Vis Sci. 2003; 80:420–30.

5. Ubels JL, Edelhauser HF, Austin KH. Healing of experimental corneal wounds treated with topically applied retinoids. Am J Ophalmol. 1983; 95:353–8.

7. Fujikawa A, Gong H, Amemiya T. Vitamin E prevents changes in the cornea and conjunctiva due to vitamin A deficiency. Graefes Arch Clin Exp Ophthalmol. 2003; 241:287–97.

8. Young E. The anti-inflammatory effects of heparin and related compounds. Thromb Res. 2008; 122:743–52.

9. Rabenstein DL. Heparin and heparin sulfate: structure and function. Nat Prod Rep. 2002; 19:312–31.
10. Upchurch GR, Valeri CR, Khuri SF, et al. Effect of heparin on fibrinolytic activity and platelet function in vivo. Am J Physiol. 1996; 271:528–34.
11. Bron AJ, Evans VE, Smith JA. Grading of corneal and conjunctival staining in the context of other dry eye tests. Cornea. 2003; 22:640–50.

12. Schiffman RM, Christianson MD, Jacobsen G, et al. Reliability and validity of the ocular surface disease index. Arch Ophthalmol. 2000; 118:615–21.

13. Her J, Yu SI, Seo SG. Clinical effects of various antiinflammatory therapies in dry eye syndrome. J Korean Ophthalmol Soc. 2006; 47:1901–10.
14. Fareed J, Walenga JM, Hoppensteadt , Messmore HL. Studies on the profibrinolytic actions of heparin and its function. Semin Thromb Hemost. 1985; 11:199–207.
15. Mousa S, Fareed J, Iqbal O, Kaiser B. Tissue factor pathway inhibitor in thrombosis and beyond. Methods Mol Med. 2004; 93:133–55.

16. Kumar R, Katare OP. Lecithin organogels as a potential phospholi-pid-structured system for topical drug delivery. AAPS Pharm Sci Tech. 2005; 6:298–310.
17. Filion MC, Phillips NC. Anti-inflammatory activity of cationic lipids. Br J Pharmacol. 1997; 122:551–7.

18. Alving CR. Immunologic aspects of liposomes: presentation and processing of liposomal protein and phospholipid antigens. Biochim. Biophy. Acta. 1992; 1113:307–22.
19. Ramos CG, Fernandes D, Charão CT, et al. Apoptotic mimicry: phosphatidylserine liposomes reduce inflammation through activation of peroxisome proliferator-activated receptors (PPARs) in vivo. Br Pharmacol. 2007; 151:844–50.

Figure 1.
Changes in ocular surface disease index score (A), Tear breakup time (B), Schirmer test (C) and Oxford grading (D) before and 1, 3 months after treatment with Hylo-Parin® and Tears Again®.
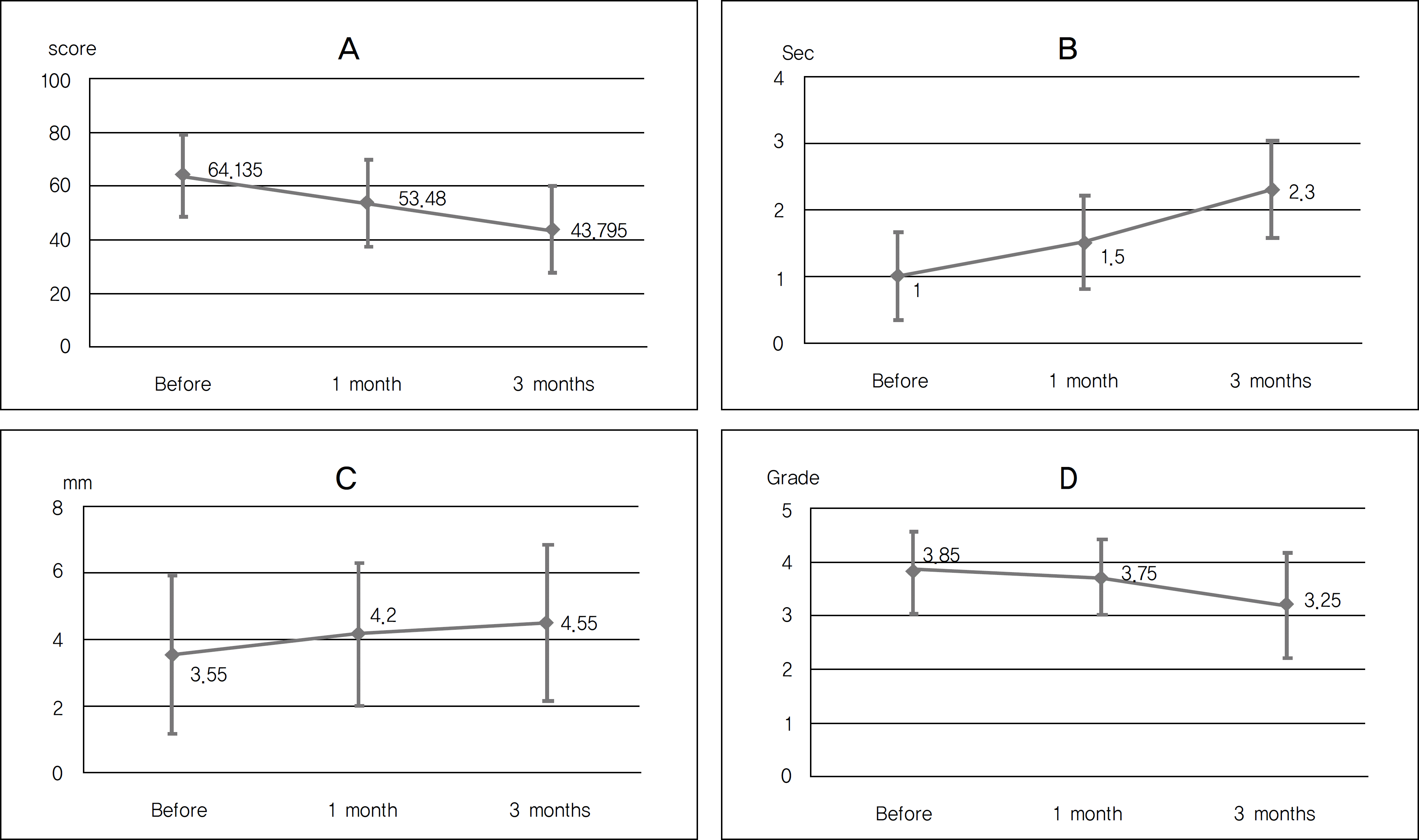
Figure 2.
Amounts of BUT change in BUT 20 eyes. After treatment for 3 months, BUT increased by 2 seconds in 9 eyes and 1 second in 6 eyes. There was no interval change in BUT in 5 eyes.
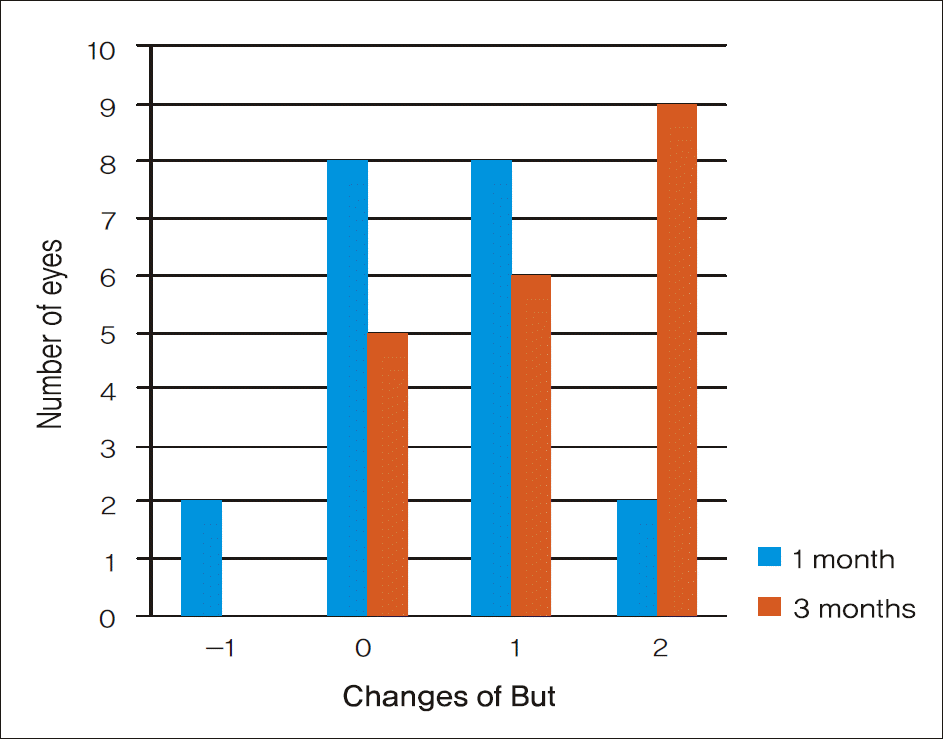
Figure 3.
Patient with keratoconjunctivitis sicca and filamentary keratitis were treated with Tears Again® and Hylo-Parin®. The right eye shows severe corneal erosions (A). After 3-months treatment, corneal erosions are healed (B). Left eye shows severe corneal erosions and filaments (C). After 3-months treatment, corneal erosions and filaments are improved (D).
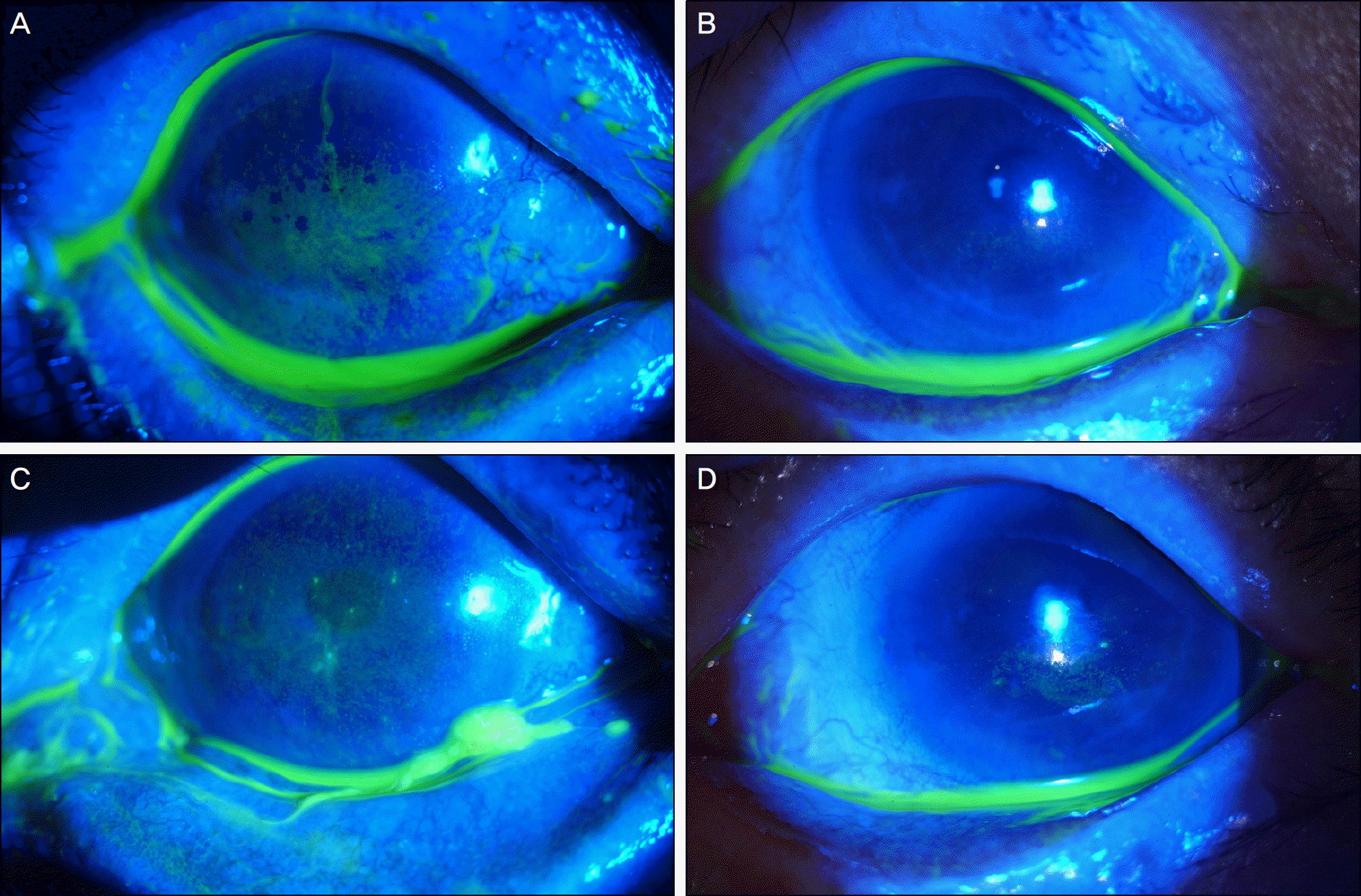
Figure 4.
In patient with a history of recurrent herpetic epithelial keratitis, corneal erosion, plaque, and filaments are observed (A). Three months after treatment with Tears Again® and Hylo-Parin®, multiple filaments and corneal erosions disappeared completely (B).
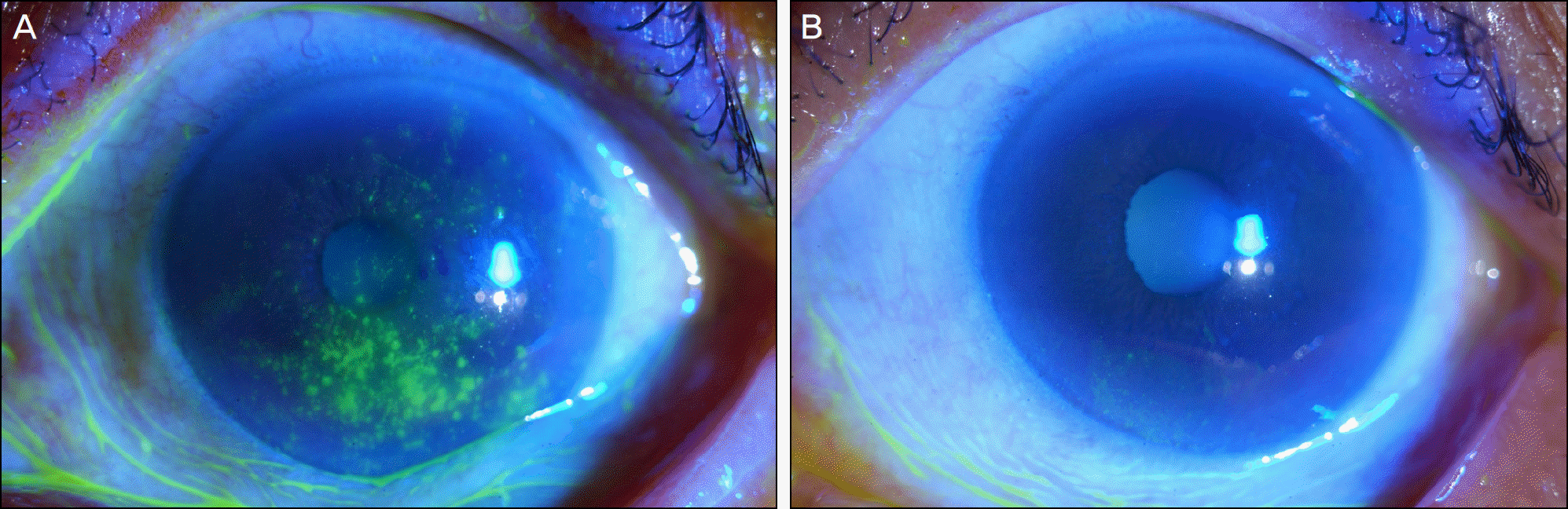
Table 1.
Constituents of Tears Again® and Hylo-Parin® (1 ml contains)
Table 2.
Demographic data




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download