Abstract
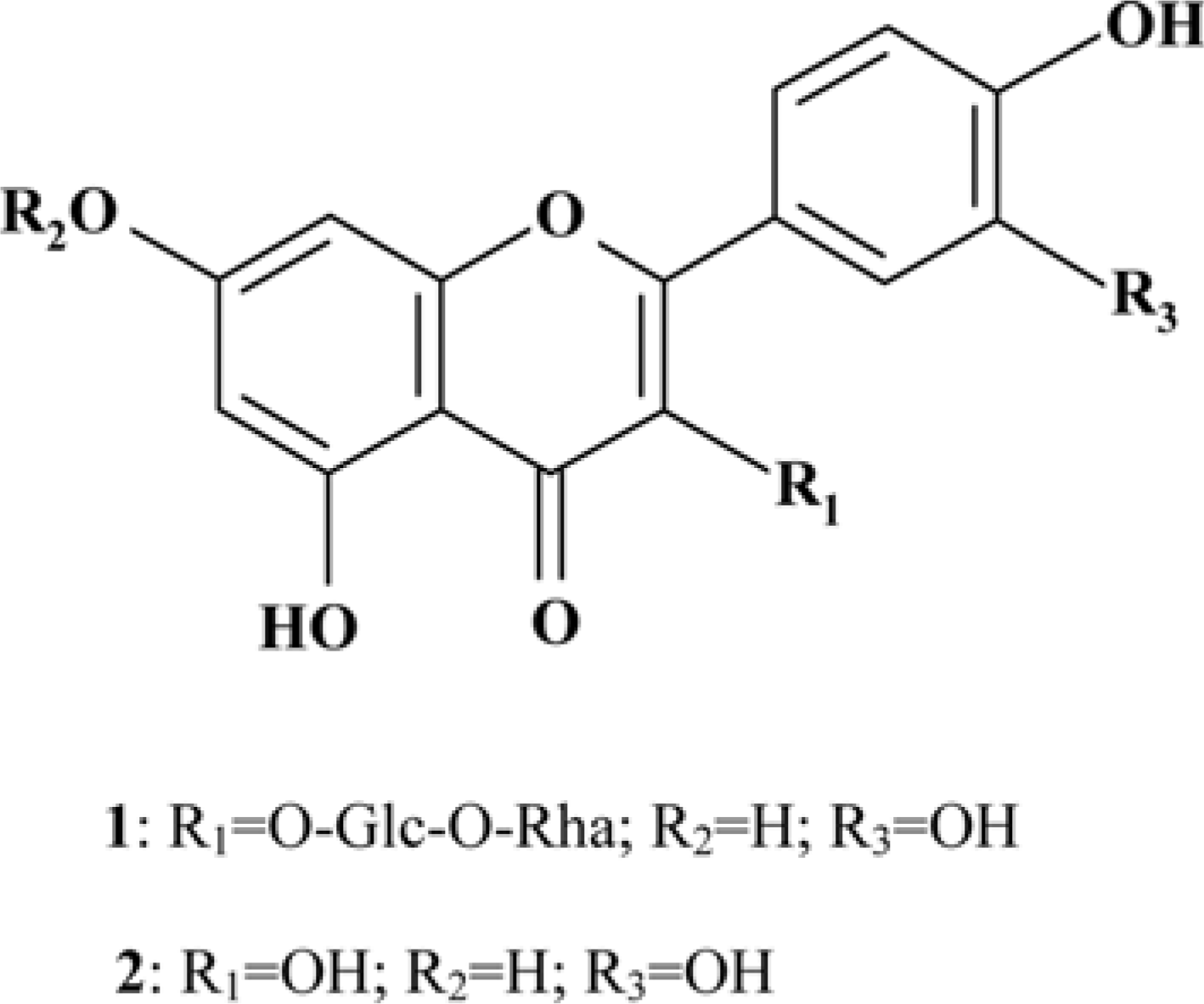
Moringa oleifera Lam (M. oleifera, Moringaceae) is a tree of the Moringaceae family that can reach a height of between 5 and 10 m. The current paper presents cytotoxic effect of M. oleifera fruits and its flavonoids 1 and 2. The viability of HCT116 human colon cancer cells were 38.5% reduced by 150 µg/mL of ethanolic extracts in a concentration-dependent manner; in addition, we observed the apoptotic features of cell shrinkage and decreased cell size. Bcl-2 family proteins were regulated as determined by Western blotting analysis, suggesting that M. oleifera fruits and their flavonoids 1 and 2 induced apoptosis through an intrinsic pathway. Based on our findings, 70% ethanolic extracts of M. oleifera fruits and flavonoids 1 and 2 might be useful as cytotoxic agents in colorectal cancer therapy.
Go to : 
References
(1). Anwar F.., Latif S.., Ashraf M.., Gilani A. H.Phytother. Res. 2007. 21:17–25.
(2). Bharali R.., Tabassum J.., Azad M. R.AsianPac. J. Cancer Prev. 2003. 4:131–139.
(3). Guevara A. P.., Vargas C.., Sakurai H.., Fujiwara Y.., Hashimoto K.., Maoka T.., Kozuka M.., Ito Y.., Tokuda H.., Nishino H.Mutat. Res. 1999. 440:181–188.
(4). Siddhuraju P.., Becker K. J.Agric. Food Chem. 2003. 51:2144–2155.
(5). Sreelatha S.., Jeyachitra A.., Padma P. R.Food Chem. Toxicol. 2011. 49:1270–1275.
(6). Budda S.., Butryee C.., Tuntipopipat S.., Rungsipipat A.., Wangnaithum S.., Lee J. S.., Kupradinun P. Asian. Pac. J. Cancer Prev. 2011. 12:3221–3228.
(8). Krishnaswamy K.., Raghuramulu N.Indian J. Med. Res. 1998. 108:167–181.
(9). Armstrong B.., Doll R.Int. J. Cancer. 1975. 15:617–631.
(10). Voorrips L. E.., Goldbohm R. A.., Van Poppel G.., Sturmans F.., Hermus R. J.., Van den Brandt P. A.Am. J. Epidemiol. 2000. 152:1081–1092.

(11). Steller H.Science. 1995. 267:1445–1449.
(12). Reed J. C.Cancer J. Sci. Am. 1998. 4:S8–S14.
(13). Reed J. C.Oncology. 2004. 18:11–20.
(14). Martinvalet D.., Zhu P.., Lieberman J.Immunity. 2005. 22:355–370.
(15). Adams J. M.., Cory S.Science. 1998. 281:1322–1326.
(16). Carmichael J.., DeGraff W. G.., Gazdar A. F.., Minna, J. D, Mitchell J. B.Cancer Res. 1987. 47:936–942.
(17). Visagie M. H.., Joubert A. M.Mol. Cell Biochem. 2011. 357:343–352.
(18). Ryu M. J.., Chung H. S.In Vitro Cell. Dev. Biol. Anim. 2015. 51:92–101.
(19). Devisetti R.., Sreerama Y. N.., Bhattacharya S. J.Food Sci. Technol. 2016. 53:649–657.
(20). Atawodi S. E.., Atawodi J. C.., Idakwo G. A.., Pfundstein B.., Haubner R.., Wurtele G.., Bartsch H.., Owen R. W. J.Med. Food. 2010. 13:710–716.
(21). Verma A. R.., Vijayakumar M.., Mathela C. S.., Rao C. V.Food Chem. Toxicol. 2009. 47:2196–2201.
(22). Manguro L. O.., Lemmen P.Nat. Prod. Res. 2007. 21:56–68.
(23). Oboh G.., Ademiluyi A. O.., Ademosun A. O.., Olasehinde T. A.., Oyeleye S. I.., Boligon A. A.., Athayde M. L.Biochem. Res. Int. 2015. 2015:175950.
(24). Karthivashan G.., Tangestani Fard M.., Arulselvan P.., Abas F.., Fakurazi S. J.Food Sci. 2013. 78:C1368–C1375.
(25). Sahakitpichan P.., Mahidol C.., Disadee W.., Ruchirawat S.., Kanchanapoom T.Phytochemistry. 2011. 72:791–795.
(26). Jintana T.., Naoto Y.., Perayot P.., Penpun W.., Tatsuro Y.., Naoki I.., Masami I.., Auayporn A.Trop. J. Pharm Res. 2017. 16:371–378.
(27). Shin S. W.., Lee Y. H.., Moon S. R.., Koo I. H.., Hong H. J.., Shin E. J.., Lee M. Y.., Park J. H.., Chung H. S. J.Kor. Soc. Appl. Biol. Chem. 2010. 53:716–723.
(28). Findley H. W.., Gu L.., Yeager A. M.., Zhou M.Blood. 1997. 89:2986–2993.
(29). Nagappan A.., Park K. I.., Park H. S.., Kim J. A.., Hong G. E.., Kang S. R.., Lee D. H.., Kim E. H.., Lee W. S.., Won C. K.., Kim G. S.Food Chem. 2012. 135:1920–1928.
(30). Oliver F. J.., de la Rubia G.., Rolli V.., Ruiz-Ruiz M. C.., de Murcia G.., Murcia J. M. J.Biol. Chem. 1998. 273:33533–33539.
Go to : 
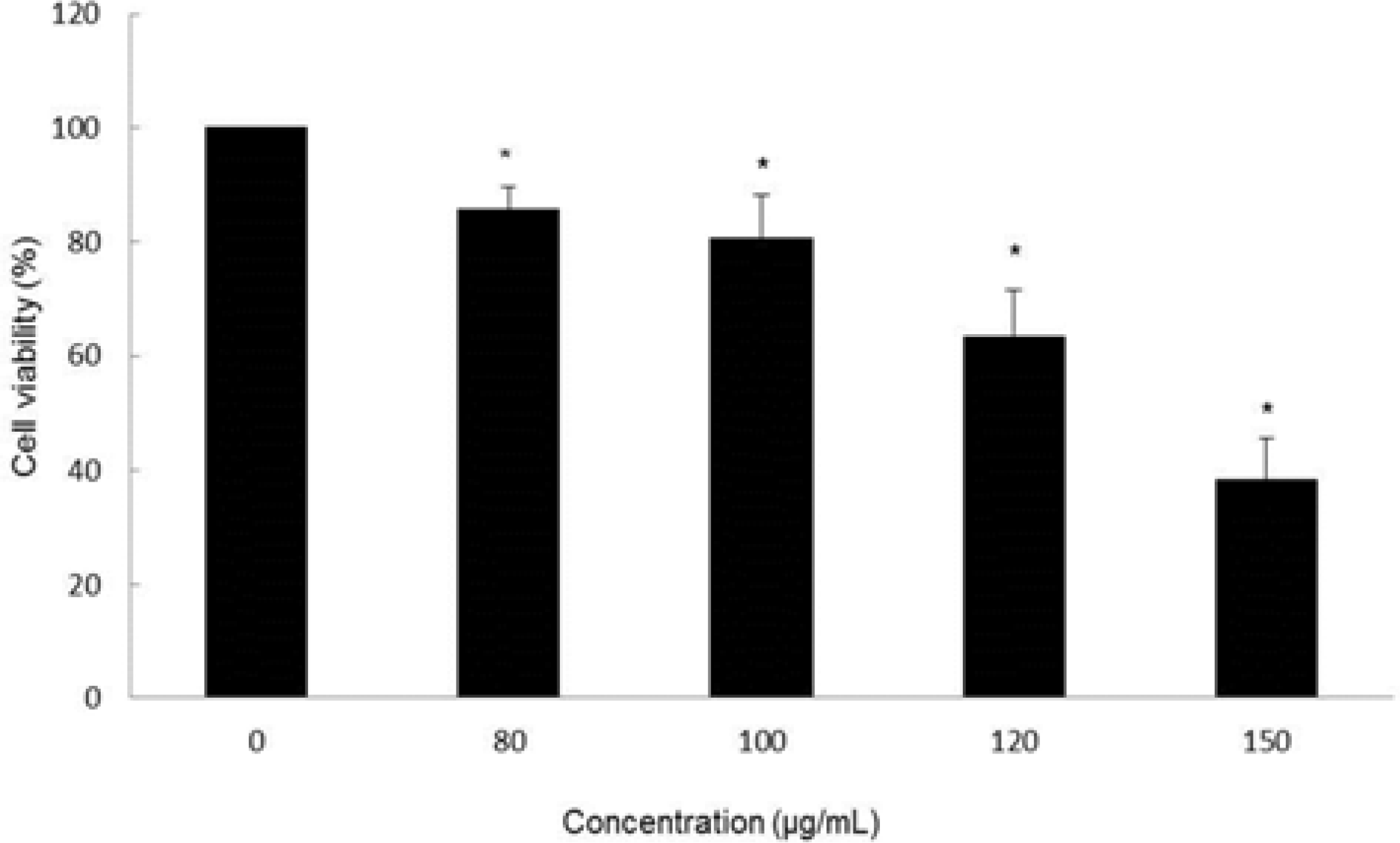 | Fig. 2.Cytotoxic effect of MOF in HCT116 cells. Cell viability at the indicated concentrations was assessed for 72 hr by MTT assay. ∗p < 0.05, significantly different from control cells. |
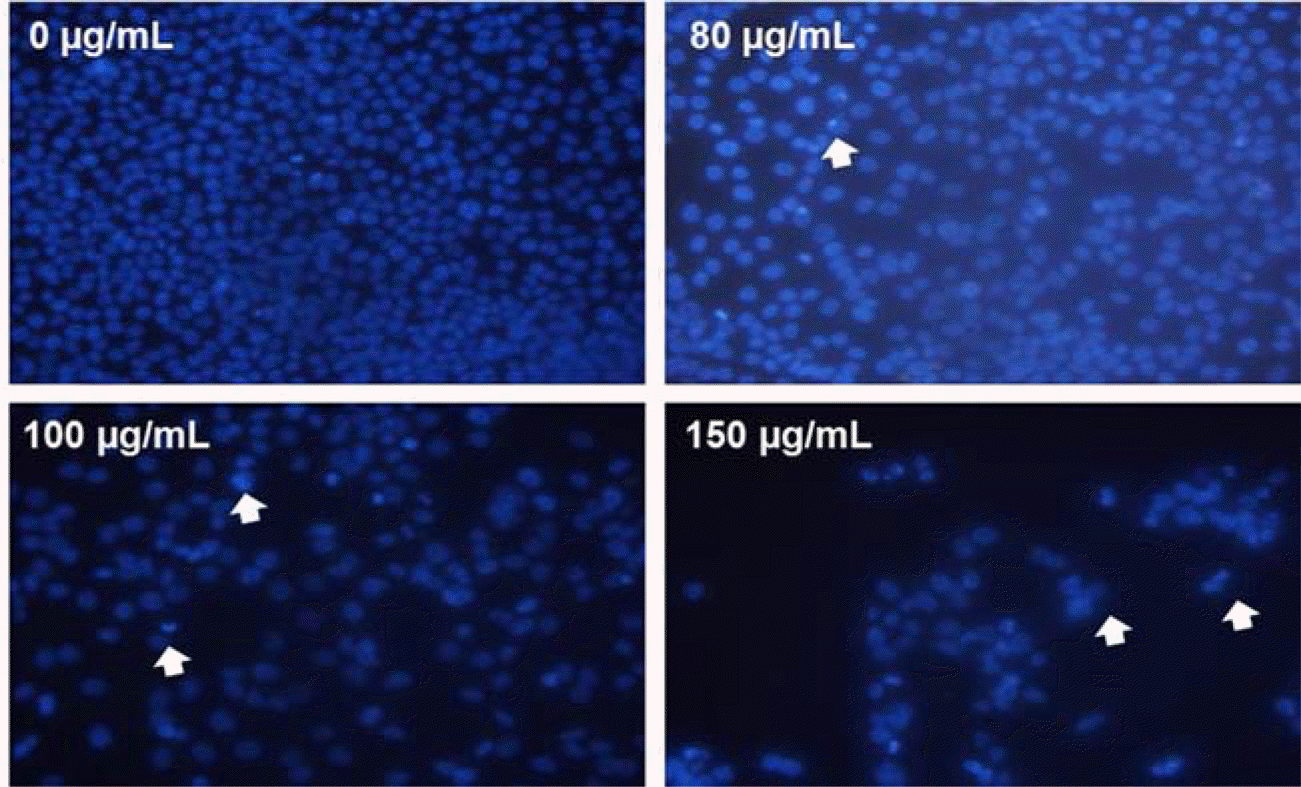 | Fig. 3.Induction of apoptosis by MOF in HCT116 cells. The formation of apoptotic bodies (arrows) in Hoechst-33258-stained cells observed by fluorescent microscopy. |
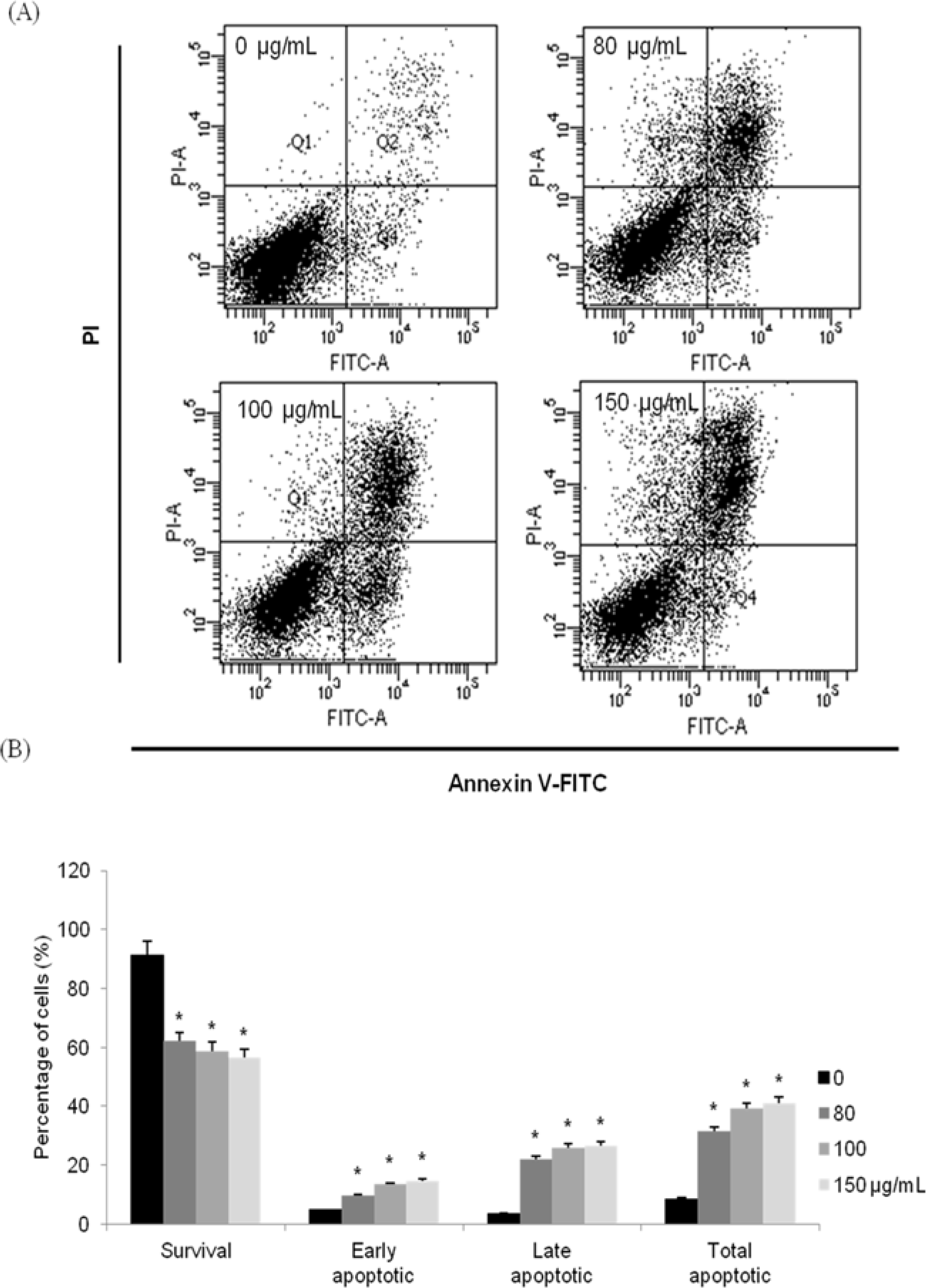 | Fig. 4.Induction of apoptosis by MOF in HCT116 cells. Flow cytometric analysis of HCT116 cells incubated with MOF for 72 hr. The right bottom quadrant represents Annexin V-stained cells (early-phase apoptotic cells). The top right quadrant represents PI- and Annexin V-stained cells (late-phase apoptotic cells). ∗p < 0.05, significantly different from control cells. |
 | Fig. 5.Regulation of Bcl-2 family in MOF-treated HCT116 cells. Equal amounts of cell lysates were electrophoresed, and Bax and Bcl-2 expression form were detected by Western blotting analysis with corresponding antibodies. ∗p < 0.05, significantly different from control cells. |
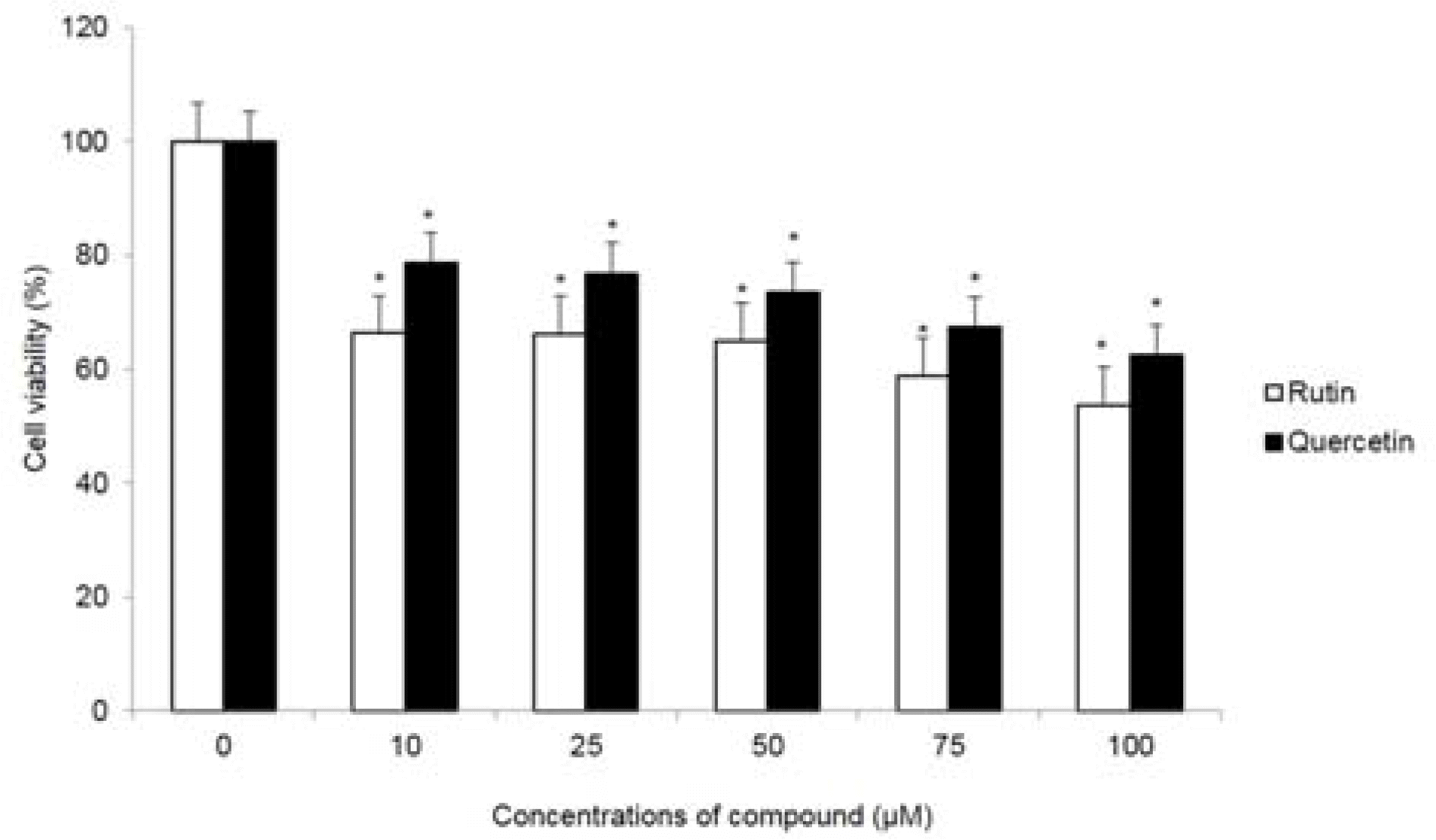 | Fig. 6.Cytotoxic effect of compounds 1 and 2 in HCT116 cells. Cell viability at the indicated concentrations was assessed for 72 hr by MTT assay. ∗p < 0.05, significantly different from control cells. |
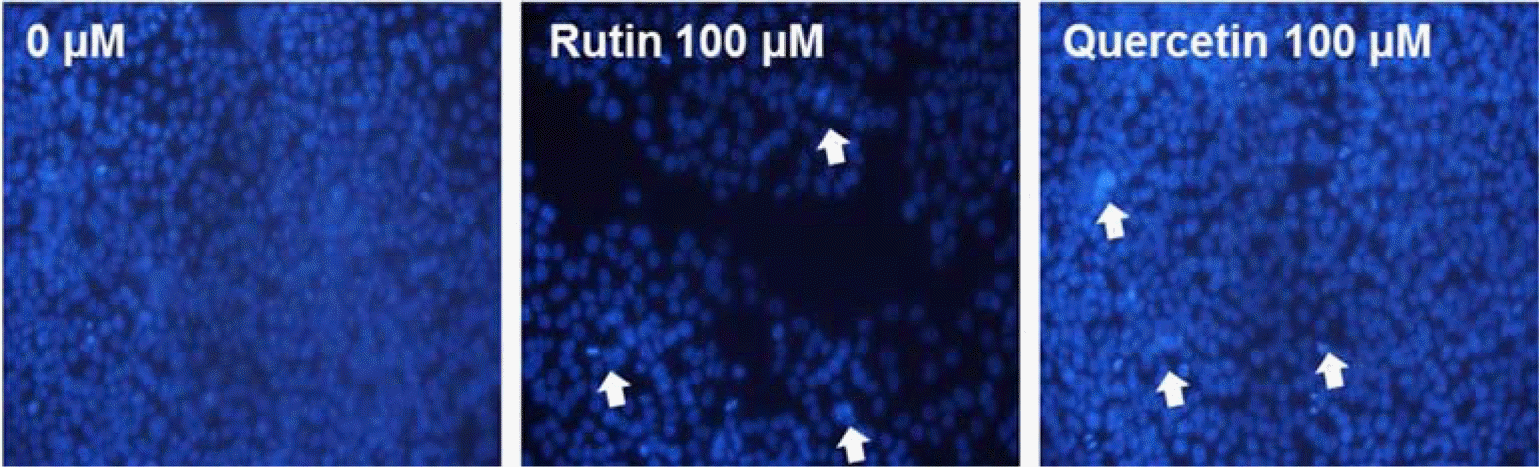 | Fig. 7.Induction of apoptosis by compounds 1 and 2 in HCT116 cells. The formation of apoptotic bodies (arrows) in Hoechst-33258-stained cells observed by fluorescent microscopy. |
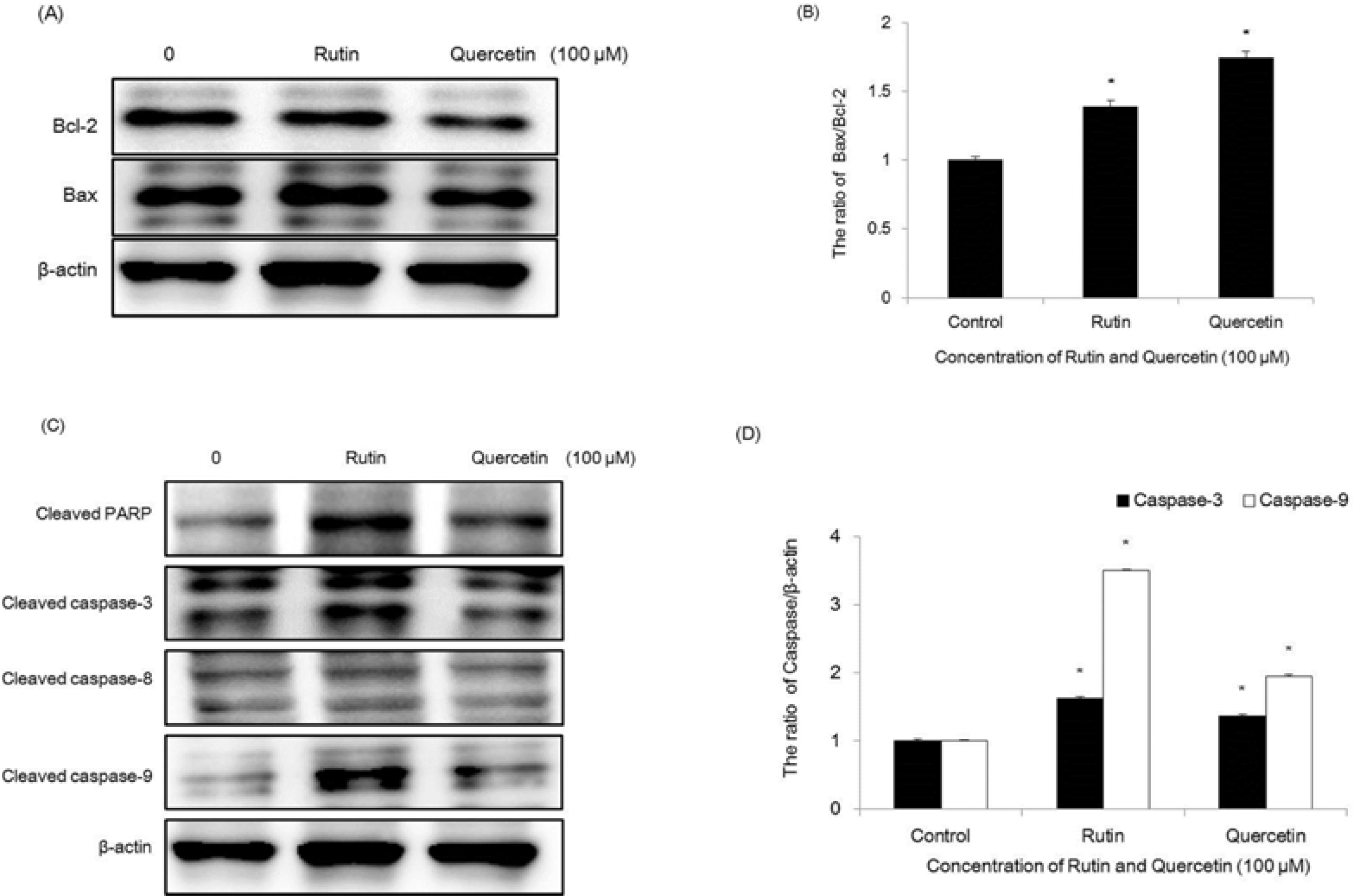 | Fig. 8.Regulation of Bcl-2 family on compounds 1 and 2-treated HCT116 cells. Equal amounts of cell lysates were electrophoresed, and Bax and Bcl-2 expression form were detected by Western blotting analysis with corresponding antibodies. ∗p < 0.05, significantly different from control cells. |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download