INTRODUCTION
MATERIALS AND METHODS
Inclusion and exclusion criteria
Search methods for identification of studies
MEDLINE via PubMed (searched on 3/13/2017) limited to English language and Humans: (“Adrenal Cortex Hormones” [Mesh] OR “adrenal cortex hormone*” OR corticosteroid* OR glucocorticoid* OR steroi* OR etodolac OR dexamethasone OR Demeclocycline OR Prednisolone OR Prednisone OR Triamcinolone Acetonide) AND (Dental pulp disease OR irreversible pulpitis OR endodontic* OR root canal) AND pain
The Web of Science and The Cochrane Library (searched on 3/13/2017). The search strategy was: (“adrenal cortex hormone*” OR corticosteroid* OR glucocorticoid* OR steroid* OR etodolac OR dexamethasone OR Demeclocycline OR Prednisolone OR Prednisone OR Triamcinolone Acetonide) AND (Dental pulp disease OR irreversible pulpitis OR endodontic* OR root canal) AND pain AND random*
-
EMBASE Library (searched on 3/13/2017) search strategy:
1) ‘adrenal cortex hormone*’ OR corticosteroid* OR glucocorticoid* OR steroid* OR etodolac OR dexamethasone OR demeclocycline OR prednisolone OR prednisone OR triamcinolone
2) Dental pulp disease
3) irreversible pulpitis
4) endodontic
5) root canal
6) Pain
7) #2 or #3 or #4 or #5
8) #1 and #6 and #7
9) Random*
10) #8 and #9
Selection of Studies and Data Extraction
Measures of Treatment Effect
Levels of evidence and summary of the review findings
RESULTS
1. Results of the search
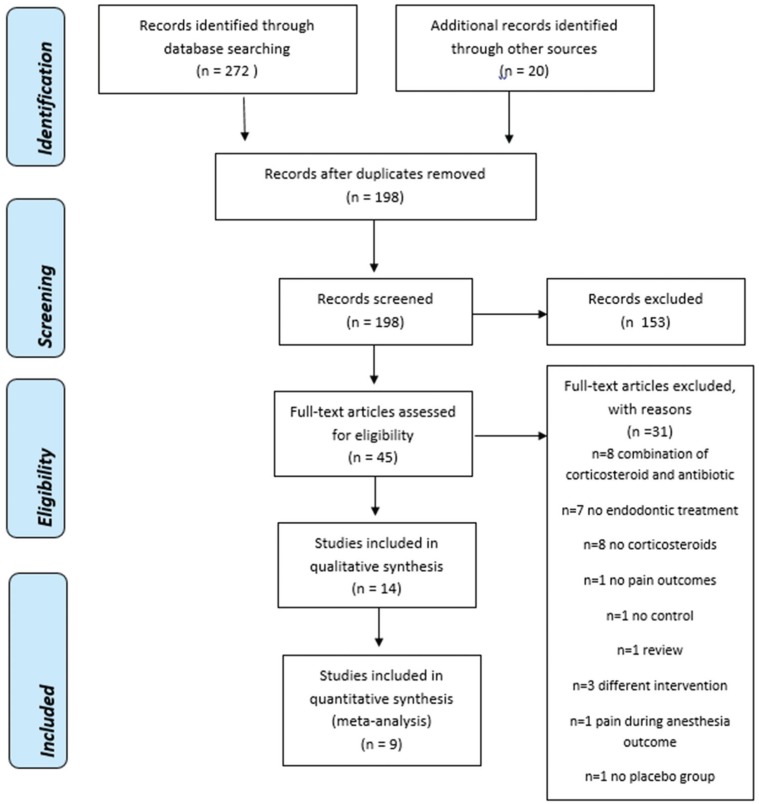 | Fig. 1PRISMA Flow Diagram [41]. |
2. Included Studies
Study design
Table 1
Summary of eligible RCT studies
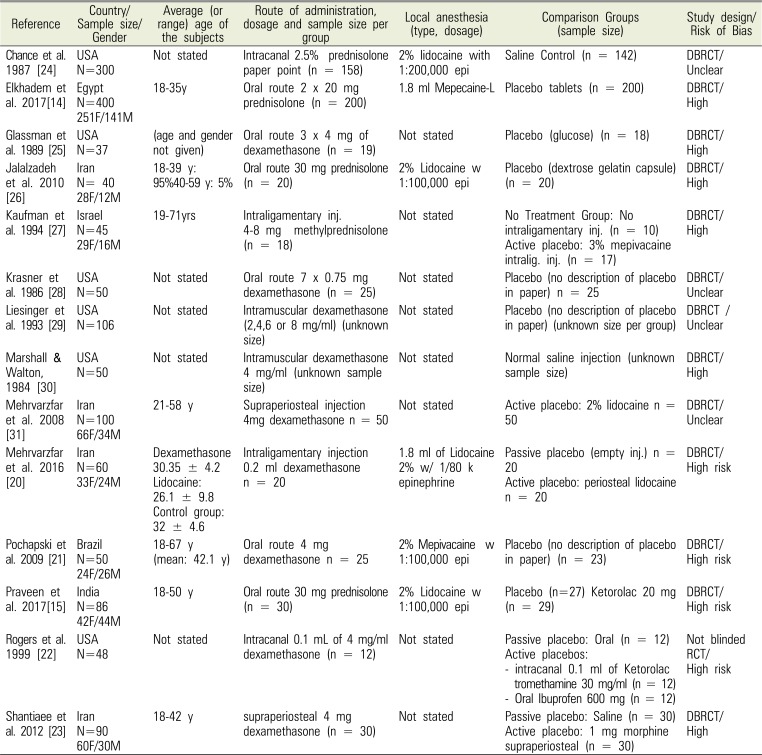
| Reference | Country/Sample size/Gender | Average (or range) age of the subjects | Route of administration, dosage and sample size per group | Local anesthesia (type, dosage) | Comparison Groups (sample size) | Study design/Risk of Bias |
|---|---|---|---|---|---|---|
| Chance et al. 1987 [24] | USA | Not stated | Intracanal 2.5% prednisolone paper point (n = 158) | 2% lidocaine with 1:200,000 epi | Saline Control (n = 142) | DBRCT/Unclear |
| N=300 | ||||||
| Elkhadem et al. 2017[14] | Egypt | 18–35y | Oral route 2 × 20 mg prednisolone (n = 200) | 1.8 ml Mepecaine-L | Placebo tablets (n = 200) | DBRCT/High |
| N=400 | ||||||
| 251F/141M | ||||||
| Glassman et al. 1989 [25] | USA | (age and gender not given) | Oral route 3 × 4 mg of dexamethasone (n = 19) | Not stated | Placebo (glucose) (n = 18) | DBRCT/High |
| N=37 | ||||||
| Jalalzadeh et al. 2010 [26] | Iran | 18–39 y: 95% 40–59 y: 5% | Oral route 30 mg prednisolone (n = 20) | 2% Lidocaine w 1:100,000 epi | Placebo (dextrose gelatin capsule) (n = 20) | DBRCT/High |
| N= 40 | ||||||
| 28F/12M | ||||||
| Kaufman et al. 1994 [27] | Israel | 19–71yrs | Intraligamentary inj. 4-8 mg methylprednisolone (n = 18) | Not stated | No Treatment Group: No intraligamentary inj. (n = 10) Active placebo: 3% mepivacaine intralig. inj. (n = 17) | DBRCT/High |
| N=45 | ||||||
| 29F/16M | ||||||
| Krasner et al. 1986 [28] | USA | Not stated | Oral route 7 × 0.75 mg dexamethasone (n = 25) | Not stated | Placebo (no description of placebo in paper) n = 25 | DBRCT/Unclear |
| N=50 | ||||||
| Liesinger et al. 1993 [29] | USA | Not stated | Intramuscular dexamethasone (2,4,6 or 8 mg/ml) (unknown size) | Not stated | Placebo (no description of placebo in paper) (unknown size per group) | DBRCT /Unclear |
| N=106 | ||||||
| Marshall & Walton, 1984 [30] | USA | Not stated | Intramuscular dexamethasone 4 mg/ml (unknown sample size) | Not stated | Normal saline injection (unknown sample size) | DBRCT/High |
| N=50 | ||||||
| Mehrvarzfar et al. 2008 [31] | Iran | 21–58 y | Supraperiosteal injection 4mg dexamethasone n = 50 | Not stated | Active placebo: 2% lidocaine n = 50 | DBRCT/Unclear |
| N=100 | ||||||
| 66F/34M | ||||||
| Mehrvarzfar et al. 2016 [20] | Iran | Dexamethasone 30.35 ± 4.2 Lidocaine: 26.1 ± 9.8 Control group: 32 ± 4.6 | Intraligamentary injection 0.2 ml dexamethasone n = 20 | 1.8 ml of Lidocaine 2% w/ 1/80 k epinephrine | Passive placebo (empty inj.) n = 20 | DBRCT/High risk |
| N=60 | Active placebo: periosteal lidocaine n = 20 | |||||
| 33F/24M | ||||||
| Pochapski et al. 2009 [21] | Brazil | 18–67 y (mean: 42.1 y) | Oral route 4 mg dexamethasone n = 25 | 2% Mepivacaine w 1:100,000 epi | Placebo (no description of placebo in paper) (n = 23) | DBRCT/High risk |
| N=50 | ||||||
| 24F/26M | ||||||
| Praveen et al. 2017[15] | India | 18–50 y | Oral route 30 mg prednisolone (n = 30) | 2% Lidocaine w 1:100,000 epi | Placebo (n=27) Ketorolac 20 mg (n = 29) | DBRCT/High risk |
| N=86 | ||||||
| 42F/44M | ||||||
| Rogers et al. 1999 [22] | USA | Not stated | Intracanal 0.1 mL of 4 mg/ml dexamethasone (n = 12) | Not stated | Passive placebo: Oral (n = 12) | Not blinded RCT/High risk |
| N=48 | Active placebos: | |||||
| - intracanal 0.1 ml of Ketorolac tromethamine 30 mg/ml (n = 12) | ||||||
| - Oral Ibuprofen 600 mg (n = 12) | ||||||
| Shantiaee et al. 2012 [23] | Iran | 18–42 y | supraperiosteal 4 mg dexamethasone (n = 30) | Not stated | Passive placebo: Saline (n = 30) | DBRCT/High |
| N=90 | Active placebo: 1 mg morphine supraperiosteal (n = 30) | |||||
| 60F/30M |
Population
Table 2
Inclusion criteria and side effects reported
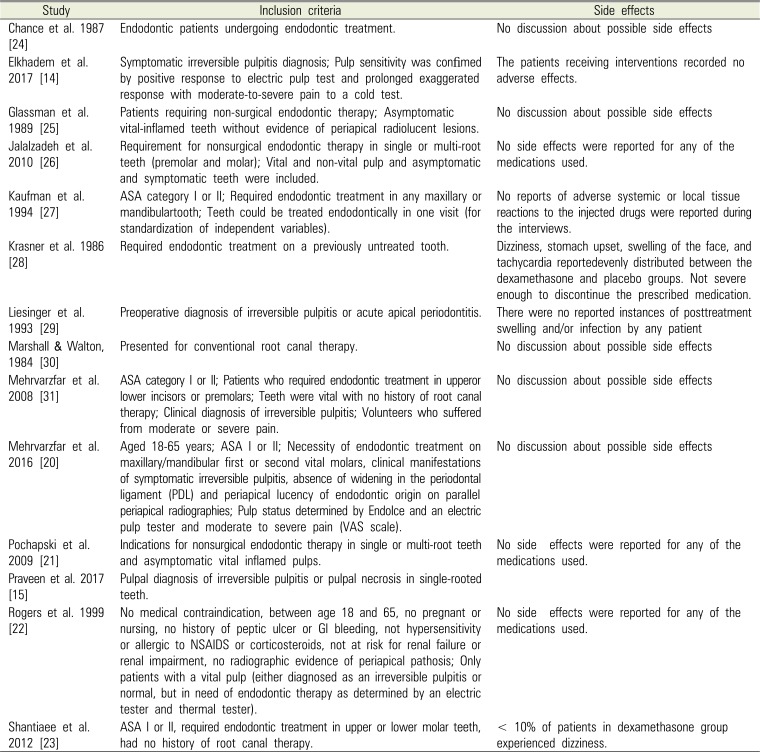
| Study | Inclusion criteria | Side effects |
|---|---|---|
| Chance et al. 1987 [24] | Endodontic patients undergoing endodontic treatment. | No discussion about possible side effects |
| Elkhadem et al. 2017 [14] | Symptomatic irreversible pulpitis diagnosis; Pulp sensitivity was confimed by positive response to electric pulp test and prolonged exaggerated response with moderate-to-severe pain to a cold test. | The patients receiving interventions recorded no adverse effects. |
| Glassman et al. 1989 [25] | Patients requiring non-surgical endodontic therapy; Asymptomatic vital-inflamed teeth without evidence of periapical radiolucent lesions. | No discussion about possible side effects |
| Jalalzadeh et al. 2010 [26] | Requirement for nonsurgical endodontic therapy in single or multi-root teeth (premolar and molar); Vital and non-vital pulp and asymptomatic and symptomatic teeth were included. | No side effects were reported for any of the medications used. |
| Kaufman et al. 1994 [27] | ASA category I or II; Required endodontic treatment in any maxillary or mandibulartooth; Teeth could be treated endodontically in one visit (for standardization of independent variables). | No reports of adverse systemic or local tissue reactions to the injected drugs were reported during the interviews. |
| Krasner et al. 1986 [28] | Required endodontic treatment on a previously untreated tooth. | Dizziness, stomach upset, swelling of the face, and tachycardia reportedevenly distributed between the dexamethasone and placebo groups. Not severe enough to discontinue the prescribed medication. |
| Liesinger et al. 1993 [29] | Preoperative diagnosis of irreversible pulpitis or acute apical periodontitis. | There were no reported instances of posttreatment swelling and/or infection by any patient |
| Marshall & Walton, 1984 [30] | Presented for conventional root canal therapy. | No discussion about possible side effects |
| Mehrvarzfar et al. 2008 [31] | ASA category I or II; Patients who required endodontic treatment in upperor lower incisors or premolars; Teeth were vital with no history of root canal therapy; Clinical diagnosis of irreversible pulpitis; Volunteers who suffered from moderate or severe pain. | No discussion about possible side effects |
| Mehrvarzfar et al. 2016 [20] | Aged 18–65 years; ASA I or II; Necessity of endodontic treatment on maxillary/mandibular first or second vital molars, clinical manifestations of symptomatic irreversible pulpitis, absence of widening in the periodontal ligament (PDL) and periapical lucency of endodontic origin on parallel periapical radiographies; Pulp status determined by EndoIce and an electric pulp tester and moderate to severe pain (VAS scale). | No discussion about possible side effects |
| Pochapski et al. 2009 [21] | Indications for nonsurgical endodontic therapy in single or multi-root teeth and asymptomatic vital inflamed pulps. | No side effects were reported for any of the medications used. |
| Praveen et al. 2017 [15] | Pulpal diagnosis of irreversible pulpitis or pulpal necrosis in single-rooted teeth. | |
| Rogers et al. 1999 [22] | No medical contraindication, between age 18 and 65, no pregnant or nursing, no history of peptic ulcer or GI bleeding, not hypersensitivity or allergic to NSAIDS or corticosteroids, not at risk for renal failure or renal impairment, no radiographic evidence of periapical pathosis; Only patients with a vital pulp (either diagnosed as an irreversible pulpitis or normal, but in need of endodontic therapy as determined by an electric tester and thermal tester). | No side effects were reported for any of the medications used. |
| Shantiaee et al. 2012 [23] | ASA I or II, required endodontic treatment in upper or lower molar teeth, had no history of root canal therapy. | < 10% of patients in dexamethasone group experienced dizziness. |
Intervention
Intracanal medication: 0.1 ml of 4 mg/ml dexamethasone or 2.5% prednisolone [2224];
Oral route: 1 × or 3 × 4 mg dexamethasone or 30–40 mg prednisolone or 7 × 7.5 mg dexamethasone or 4 mg dexamethasone [21252628];
Intraligamentary injection: 4–8 mg slow release prednisolone or 0.2 ml dexamethasone [2027];
Intramuscular injection: 2–4–6–8 mg/mL dexamethasone or 4 mg/ml [2930];
Comparison
Outcomes
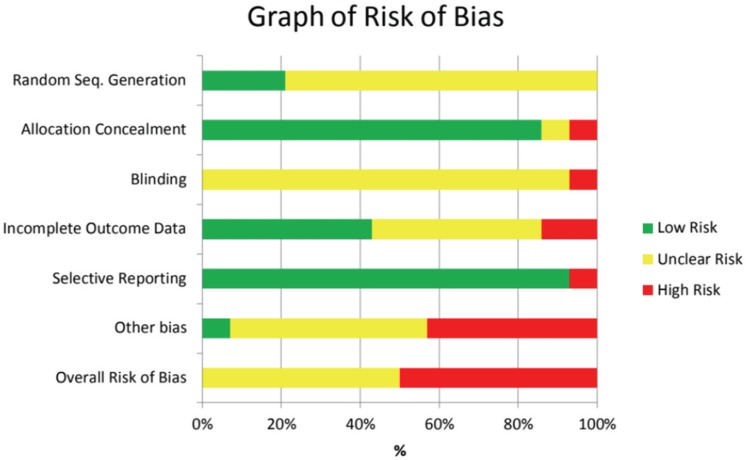
3. Risk of bias in included studies
Table 3
Summary of risk of bias for eligible RCT studies
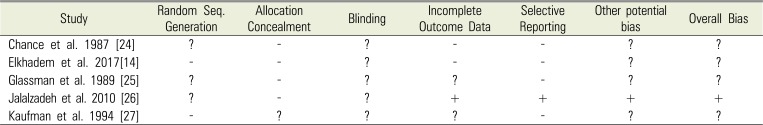
| Study | Random Seq. Generation | Allocation Concealment | Blinding | Incomplete Outcome Data | Selective Reporting | Other potential bias | Overall Bias |
|---|---|---|---|---|---|---|---|
| Chance et al. 1987 [24] | ? | - | ? | - | - | ? | ? |
| Elkhadem et al. 2017[14] | - | - | ? | - | - | ? | ? |
| Glassman et al. 1989 [25] | ? | - | ? | ? | - | ? | ? |
| Jalalzadeh et al. 2010 [26] | ? | - | ? | + | + | + | + |
| Kaufman et al. 1994 [27] | - | ? | ? | ? | - | ? | ? |
Random Sequence Generation
Allocation Concealment
Blinding
Incomplete outcome data
Selective reporting
Other bias
4. Effects of interventions
Primary outcome (post-treatment VAS pain)
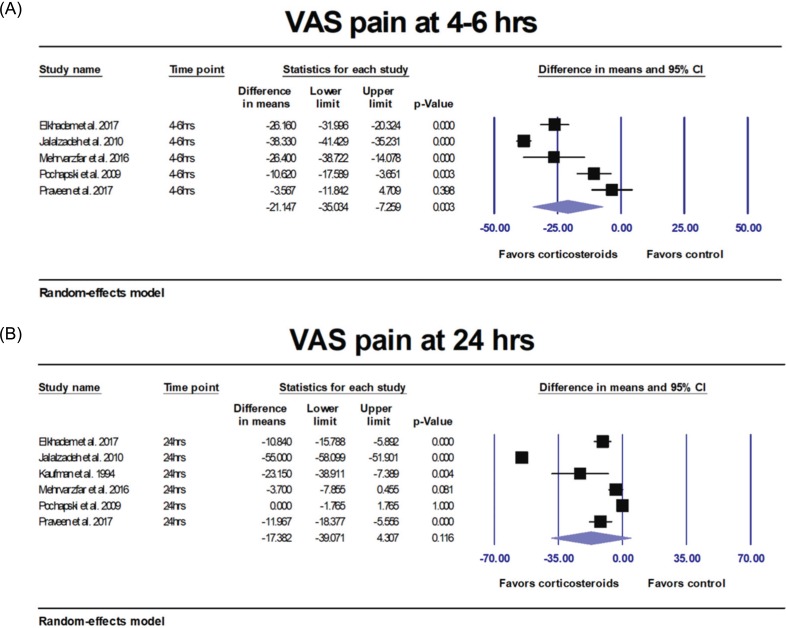 | Fig. 3(A) Results of the meta-analyses comparing corticosteroids versus controls. VAS pain was significantly decreased (P = 0.003) at 4–6 hours after IANB. (B) Results of the meta-analyses comparing corticosteroids versus controls. VAS pain was not significantly decreased after 24 hours (P = 0.116). |
Subgroup analysis by route of administration
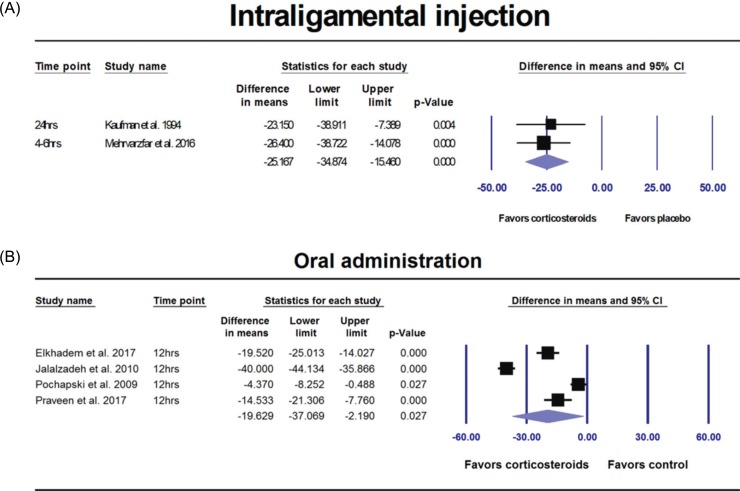 | Fig. 4(A) Results of the subgroup analyses by route of administration comparing corticosteroids versus control group. VAS pain was significantly decreased with corticosteroids delivered via intraligamental injection (P < 0.001). (B) Results of the subgroup analyses by route of administration comparing corticosteroids versus control group. VAS pain was significantly decreased with corticosteroids via oral administration (P = 0.027). |
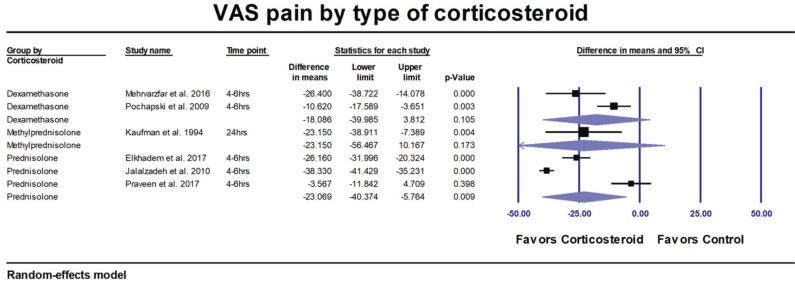
Subgroup analysis by type of corticosteroids
Likelihood of none or mild post-treatment pain
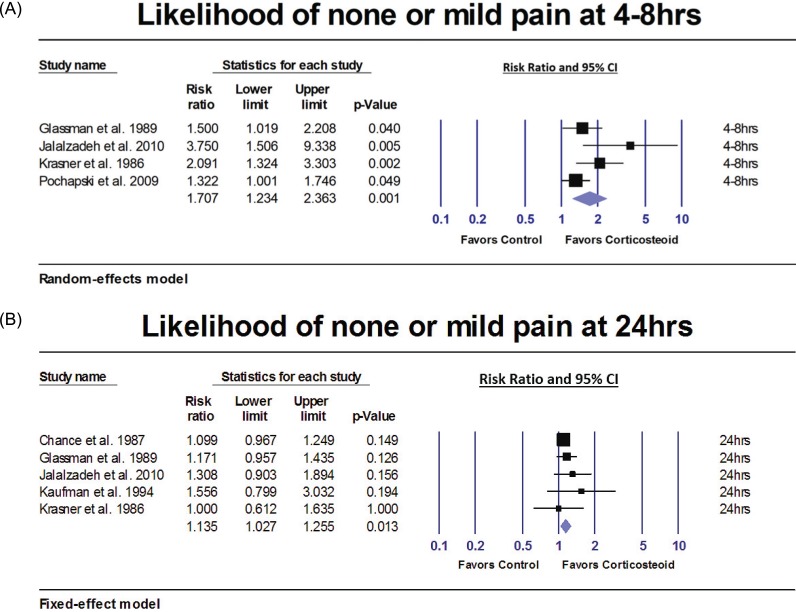 | Fig. 6A) Results of the meta-analyses comparing corticosteroids versus control group. Patients who received corticosteroids prior to IANB were 70.7% more likely to have none or mild pain 4–8 hours after IANB (P = 0.001). (B) Results of the meta-analyses comparing corticosteroids versus control group. Patients who received corticosteroids prior to IANB were 13.5% more likely to have none or mild pain 24 hours after IANB (P = 0.013) than patients in the control group. |
Symptomatic, asymptomatic patients or both
5. Adverse effects
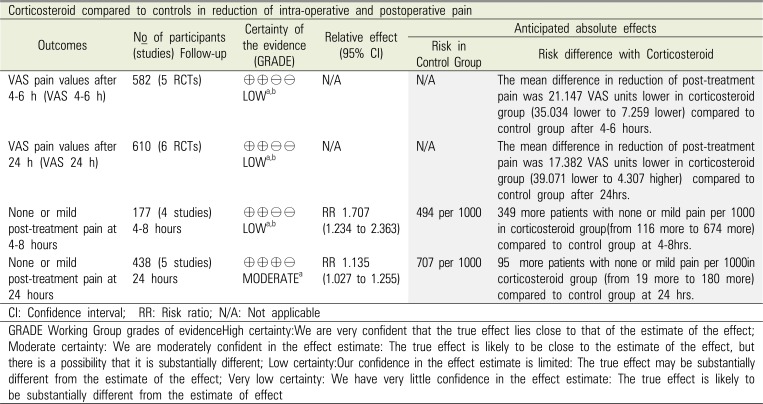
Summary of the evidence and quality of the findings (GRADE)
Table 4
Summary of the evidence and quality of the findings (GRADE)




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download