This article has been
cited by other articles in ScienceCentral.
Abstract
In this study, a formulation of Bordetella pertussis proteoliposome (PLBp), diphtheria, and tetanus toxoids and alum (DT-PLBp) was evaluated as a trivalent vaccine candidate in BALB/c mice. Vaccine-induced protection was estimated using the intranasal challenge for pertussis and enzyme-linked immunosorbent assay fvto assess serological responses for diphtheria or tetanus. Both, diphtheria-tetanus-whole cell pertussis (DTP) and diphtheria-tetanus vaccines (DT) were used as controls. Animals immunized with DT-PLBp, PLBp alone, and DTP showed total reduction of CFU in lungs 7 days after intranasal challenge. Likewise, formulations DT-PLBp, DTP, and DT elicited antibody levels ≥2 IU/mL against tetanus and diphtheria, considered protective when neutralization tests are used. Overall, results showed that combination of PLBp with tetanus and diphtheria toxoids did not affect the immunogenicity of each antigen alone.
Keywords: Bordetella pertussis, Proteoliposome, Whooping cough, Vaccines
Whooping cough, an acute respiratory disease caused by
Bordetella pertussis, still is an important cause of public health concern worldwide even in countries with high vaccination coverage [
1]. Various reasons could explain this phenomenon, among them, waning of vaccine-induced immunity has shift the incidence peak from young children to adolescents and adults, which become source of infection for unvaccinated newborns [
2].
Improvement of existing vaccines or the development of new and better ones, capable of conferring a long-lasting protection against disease has been recommended by some experts [
34]. Bacterial-derived proteoliposomes (PL) are nanoparticulate vesicular structures that contain proteins, lipids, and native lipopolysaccharide (LPS) [
56]. Their immune-stimulatory and adjuvant properties made them attractive for vaccine development against several diseases [
5]. A previous work described the obtaining of a PL derived from
B. pertussis (PLBp). Preliminary characterization of PLBp revealed the presence of LPS and some important proteins such as pertussis toxin, pertactin, and fimbriae; also,
Limulus amoebocyte lysate assay showed that PLBp has lower endotoxin level than those reported for traditional whole cell pertussis licensed vaccines. In addition, immunization with PLBp protected mice in the intracranial and intranasal challenge models [
7].
Since 1947, pertussis vaccines are administered with diphtheria and tetanus toxoids first, and later with other antigens to form combined vaccines [
8]. In this work, we evaluated the protection conferred by a combined formulation of PLBp with diphtheria and tetanus toxoids in BALB/c mice.
PLBp was obtained as described previously [
7] and formulated at 120 µg/mL with 50 Lf/mL of diphtheria toxoid and 20 Lf/mL of tetanus toxoid using aluminum hydroxide (2 mg/mL) as adjuvant (diphtheria-tetanus and
B. pertussis proteoliposome [DT-PLBp]). Vaccines DTP-vax (diphtheria 50 Lf/mL, tetanus 20 Lf/mL, and whole cell pertussis 32 OU/mL) and VA-DIFTET (diphtheria 50 Lf/mL and tetanus 20 Lf/mL) produced at Finlay Institute, Cuba, were also used in this study as controls. In-house diphtheria and tetanus reference sera were obtained and supplied by the Reference Material Department from Finlay Institute.
Female BALB/c mice, 3-4 weeks old, were supplied by the National Center for Laboratory Animals Breeding (CENPALAB) from Havana, Cuba with their health certificates. Animals were housed at the Finlay Institute animal facility and kept following the Canadian Council directions for laboratory animal experiments. All experiments were performed with approval from the Finlay Institute Ethical Committee.
Groups of 18 mice were immunized subcutaneously with two doses of 125 µL of each vaccine, separated by a 3-week interval. Two weeks after the second dose, serum samples of 10 mice were obtained for the assessment of diphtheria and anti-tetanus immune response by enzyme-linked immunosorbent assay (ELISA). Briefly, microplates were coated with diphtheria and tetanus toxoids (2Lf/mL) in carbonate-bicarbonate buffer (pH 9.6) and incubated overnight, at 2-8℃. After washing, 2-fold dilutions of the references and samples sera in phosphate buffered saline (PBS)-Milk 3%-Tween 20 0.05%, starting in 1/400 and 1/800, respectively, were prepared and added to the plates. One-hour later, a working dilution of an anti-mouse IgG conjugated to horseradish peroxidase was applied. After 1-hour incubation, a substrate solution (ortho-phenylendiamine in citrate buffer) was added and the plates were incubated in darkness, for 30 minutes, at room temperature. Reaction was stopped with sulphuric acid 1 M and absorbances were read at 492 nm. Results were expressed in International Units (IU/mL) as the arithmetic means±standard deviation. An antibody level equal or higher than 2 IU/mL was considered as protective for both antigens as was seen in correlation studies made between these ELISA and the
in vivo toxin neutralization test [
9].
The intranasal challenge was performed 2 weeks after the last immunization. Each mouse was slightly anaesthetized with ether and then 50 µL (25 µL per nostril) of PBS containing approximately 5×10
6-10
7 CFU of
B. pertussis strain 18323 were administrated intranasally. Lung extraction for CFU counting were done two hours and 7 days post-challenge (4 mice per time) as described by Guiso et al. [
10]. Results were expressed as the arithmetic means±standard deviation of log10 of the CFU/g of lung for each group of mice at each extraction time after challenge.
The comparison of arithmetic means of the groups was carried out by an analysis of simple variance and Tukey's multiple comparison test was used to compare groups with a confidence level of 95% (p<0.05) (GraphPad Prisma 5, La Jolla, CA, USA).
Immune response against tetanus and diphtheria elicited by each group is showed in
Figs. 1 and
2, respectively. Groups immunized with diphtheria-tetanus vaccine (DT), DTP, and DT-PLBp elicited antibody levels higher to 2 IU/mL for both antigens. These antibody levels are related to their ability to neutralize the tetanus and diphtheria toxins, as shown by the correlation studies performed between the serological methods and the toxin neutralization assays [
9]. Thus, it was demonstrated that the immunized vaccines generated protective antibodies and the PLBp did not affected the immunogenicity of the toxoids.
As observed in
Table 1, seven days after intranasal challenge, no CFU were recovered in lungs from mice of groups immunized with PLBp, DT-PLBp, and DTP vaccine. On the other hand, in the group immunized with the DT, the number of CFU recovered from lungs was not significantly different (p>0.05) from CFU recovered 2 hours after challenge. Same results were observed by our group previously, using PLBp and DTP vaccine [
7].
Triple combination of diphtheria-tetanus toxoids and pertussis component (whole cell or acellular) is one of the most used vaccines worldwide (131 countries with ≥90% coverage in 2012) in a 3-dose primary series and booster in the second year of live [
11]. In addition, the use of reduced diphtheria/acellular pertussis and tetanus vaccines has been recommended for additional boosters in adolescents and adults in order to decrease incidence in these age groups [
12]. On the other hand, there is now convincing evidence available that the current acellular pertussis vaccines does not provide an adequate protective immunity and, the bacteria is adapting in order to evade the vaccines pressure [
13]. This calls for the development of new and improved pertussis vaccines as a long-term control measure. Ideally, this new vaccine should have to (1) induce a pattern of immune response similar to natural infection, (2) elicit a long-lasting immunity, (3) show a reactogenic profile better than traditional whole cell pertussis vaccines, (4) be multi-antigenic in order to elude the vaccine pressure, and (5) be affordable for both developed and developing countries. In that matter, a
Bordetella pertussis derived proteoliposome may represent a good approach [
57]. Results obtained here indicated that combination of these antigens did not affect the protective capacity of PLBp and the immune response elicited by diphtheria-tetanus toxoids, showing its potential use in a trivalent formulation. Further studies are needed in order to elucidate duration of protection and the immune response pattern induced by PLBp as well as deepen in its antigenic composition, but these results encourage us to continue in that direction.
Figures and Tables
Fig. 1
Immune response anti-tetanus toxoid by enzyme-linked immunosorbent assay. Groups were immunized with two doses (3 weeks apart) and 2 weeks after the second dose, blood samples were taken from 10 mice per group. DTP, diphtheria-tetanus-whole cell pertussis vaccine; DT, diphtheria-tetanus vaccine; PLBp, B. pertussis proteoliposome; DT-PLBp, diphtheria-tetanus and B. pertussis proteoliposome. Comparison of arithmetic means was carried out by simple ANOVA and confidence levels (p<0.05) by Tukey's multiple comparison test.
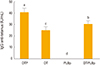
Fig. 2
Immune response anti-diphtheria toxoid by enzyme-linked immunosorbent assay. Groups were immunized with two doses (3 weeks apart) and 2 weeks after the second dose blood samples were taken from 10 mice per group. DTP, diphtheria-tetanus-whole cell pertussis vaccine; DT, diphtheria-tetanus vaccine; PLBp, B. pertussis proteoliposome; DT-PLBp, diphtheria-tetanus and B. pertussis proteoliposome. Comparison of arithmetic means was carried out by simple ANOVA and confidence levels (p<0.05) by Tukey's multiple comparison test.
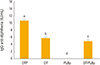
Table 1
Intranasal challenge experiments in BALB/c mice using Bordetella pertussis strain 18323

|
Bacterial recovered after challenge (log CFU/g lung±SD) |
|
2 hr |
7th day |
|
DTP |
7.26 ± 0.05 |
NR |
|
PLBp |
7.45 ± 0.10 |
NR |
|
DT-PLBp |
7.63 ± 0.02 |
NR |
|
DT |
6.95 ± 0.13 |
6.13 ± 0.02 |






 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download