Abstract
Transplantation of spermatogonial stem cells (SSCs) in experimental animal models has been used to study germ line stem cell biology and to produce transgenic animals. The species-specific recipient model preparation is important for the characterization of SSCs and the production of offspring. Here, we investigated the effects of surgically induced cryptorchidism in dog as a new recipient model for spermatogonial stem cell transplantation. Artificially unilateral or bilateral cryptorchidism was induced in ten mature male dogs by surgically returning the testis and epididymis to the abdominal cavity. The testes and epididymides were collected every week after the induction of artificial cryptorchidism (surgery) for one month. To determine the effect of surgical cryptorchidism, the seminiferous tubule diameter was measured and immunohistochemistry using PGP9.5 and GATA4 antibodies was analyzed. The diameters of the seminiferous tubules of abdominal testes were significantly reduced compared to those of the scrotal testes. Immunohistochemistry results showed that PGP9.5 positive undifferentiated spermatogonia were significantly reduced after surgical cryptorchidism induction, but there were no significant changes in GATA-4 positive sertoli cells. To evaluate the testis function recovery rate, orchiopexy was performed on two dogs after 30 days of bilateral cryptorchidism. In the orchiopexy group, SCP3 positive spermatocytes were detected, and spermatogenesis was recovered 8 weeks after orchiopexy. In this study, we provided optimum experimental conditions and time for surgical preparation of a recipient canine model for SSC transplantation. Additionally, our data will contribute to recipient preparation by using surgically induced cryptorchidism in non-rodent species.
Spermatogonial stem cells (SSCs) are the unique germline and adult stem cells responsible for sperm production by spermatogenesis. Spermatogenesis occurs throughout the life of mammalian males and comprises the differentiation and proliferation of SSCs [1]. The technique of SSC transplantation was first introduced by Brinster and colleagues in 1994 using rodents [2] and has been extensively used to study spermatogenic function in different species [34567]. SSC transplantation provides an alternative method to generate transgenic animals, and offers a new strategy for the preservation of genetically valuable individuals [8]. This technique consists of harvesting testis cells from a donor male and placing the cell population, including SSCs, into the seminiferous tubules of an infertile recipient [9].
The first key step in successful SSC transplantation is recipient preparation [1011]. In large animals, unlike in rodents, two methods have been generally used to reduce the number of endogenous SSCs in recipient testis: chemotherapy using drugs such as busulfan [12], and focal irradiation [3613]. However, the SSC-depleting dose of busulfan administration can produce systemic toxicity and cause severe bone marrow depression [1112]. Furthermore, the side effects of busulfan treatment significantly reduced the survival of exogenous SSCs and threatened the fertility of recipient animals. Focal radiation therapy can be used as an alternative to cytotoxic treatment for the depletion of endogenous germ cells. However, this method requires expensive and specialized equipment to generate therapeutic radiation and induces the calcification of seminiferous tubules in rodent [14]. Therefore, a safer and more efficient method of recipient preparation for SSC transplantation is required.
It was well known that surgical induction of cryptorchidism in experimental animals causes the rapid degeneration of germ cell in testes and disrupts spermatogenesis [1516]. By contrast, sertoli cells, which are essential for spermatogenesis, appear to be normal when exposed to the higher abdominal temperatures [17]. Despite general agreement that cryptorchidism leads to spermatogenic damage and that orchiopexy improves spermatogenesis, the extent of the damage or improvement remains obscure. To date, there has been little information regarding the effects of surgically induced cryptorchidism for preparing SSC transplant recipients in the dog. In the present study, we investigated the use of surgically induced cryptorchidism to deplete male mongrel dog testes of endogenous germ cells, a step necessary to prepare them for use as SSCs transplantation recipients.
Ten mature male mongrel dogs that were six to seven months of age and weighed 25.62±2.01 kg (mean±standard deviation) were used in the present study. All dogs were determined to be in good physical condition based upon a physical examination. Experimental dogs were kept in individual pens and received standard balanced canine diets throughout the experiment. All animals were housed for at least seven days before the day of the experiment to adapt to the environment. Animals were kept on a 12-hour light/dark cycle under controlled environmental conditions. Prior to the day of the surgery, food was withheld for at least 12 h before surgery, but the dogs were allowed free access to water. Experimental procedures were conducted in accordance with the guidelines approved by the Institutional Animal Care and Use Committee (IACUC) at the Konkuk University (approved No. KU15187).
Experimental dogs received acepromazine (0.1 mg/kg; IM) for premedication and propofol (6 mg/kg; IV) for induction of anesthesia. Endotracheal intubation was performed, and anesthesia was maintained via the inhalation of a combination of isoflurane and oxygen. Physiologic monitoring of animals during surgery and anesthesia included continuous pulse oximetry, ECG, heart and respiratory rates, and body temperature via an esophageal probe. The entire abdomen, from xiphoid to scrotum, was prepared under aseptic conditions. The urinary bladder was emptied via catheterization. Dogs were positioned in dorsal recumbency on a tilting table with the head tilted downward. To surgically induce unilateral or bilateral cryptorchidism, a 4 cm long paramedical incision was made through the skin in the inguinal region. One or two testes were pushed out through the incision, and the fascial attachment between the tail of the epididymis and the scrotum was cut (Figure 1A). The superficial and internal inguinal ring was exposed and enlarged by incising it, and the testicle was introduced into the same side abdominal cavity (Figure 1B). The inguinal ring was closed to prevent the normal descent of the testicle (Figure 1C). After suturing and disinfecting the incision sites, all animals received intramuscular cefazolin sodium (22 mg/kg) for 5 days. In the unilateral group, shamoperated scrotal testes were used as a control. Abdominal and scrotal testes were removed and decapsulated for histological and immunohistochemical analysis 7, 14, 21, and 30 days after surgery. In the orchiopexy group, two dogs underwent bilateral cryptorchidism. After 30 days, the abdominal testes were returned to the scrotum through an inguinal incision and sutured to connect the testes to the scrotum (Figure 1D). Four and eight weeks later, testes were removed and assessed as described above.
Testes and epididymis samples from unilateral cryptorchidism and orchiopexy groups were fixed in Bouin's solution (HT10132; Sigma-Aldrich, St. Louis, MO, USA) overnight at 4℃. Samples were subsequently washed in 70% to 100% (v/v) ethanol, embedded in paraffin, sliced into 5 µm thick sections using a microtome (Thermo, Barrington, IL, USA), and mounted onto glass slides. The mounted canine testis tissue was rehydrated using xylene and 100 to 50% ethanol. The nuclei and cytoplasm were stained with hematoxylin and eosin (H & E). To determine the location of PGP9.5, GATA4, and SCP3 in the testis cells, antigens were retrieved by boiling for 30 min in a tris-ethylenediaminetetraacetic acid (EDTA) solution (10 mM Tris base, 1 mM EDTA, 0.05% Tween 20, pH 9). The antigen-retrieved tissues were membrane permeabilized with PBS containing 0.1% Triton X-100 for 10 minutes. Non-specific protein binding was blocked with 2% bovine serum albumin (BSA) in PBS for 1 hour at room temperature (RT), and tissues were incubated overnight at 4℃ with the following primary antibodies: anti-PGP9.5 antibody (1:1000; 7863- 1004; AbD Serotec, Raleigh, NC, USA), anti-GATA4 antibody (1:50; SC-1237; Santa Cruz Biotechnology, Inc.; Dallas, Texas, USA), and anti-SCP3 antibody (1:50; SC-27333; Santa Cruz Biotechnology, Inc.; Dallas, Texas, USA). After washing three times with PBS, the appropriate secondary antibodies were added for two hours at room temperature. The peroxidase substrate detection kit (SK-4100; Vector Laboratories) was used for detection of the putative canine germ cell markers according to the manufacturer's instructions. For each testis, the diameter of seminiferous tubules in ten randomly selected tubular profiles that were round or nearly round, were measured under light microscopy.
The diameter of seminiferous tubules and number of PGP9.5, and GATA4 positive cells were analyzed by ANOVA and Tukey,s tests using Graphpad Prism 5 software (Graphpad, LaJolla, CA, USA). with P<0.05 considered statistically significant. The mean and standard deviation (SD) were calculated for each value.
To evaluate the effect of surgically induced unilateral cryptorchidism, H & E staining was performed. The histological appearance and diameter of the seminiferous tubules in the scrotal and cryptorchid testes of dogs 7, 12, 21, and 30 days following surgical induction of cryptorchidism was analyzed (Figure 2). No significant change in seminiferous tubule diameter was observed in the scrotal testes measured at different days (Figure 2). Surgical induction of cryptorchidism significantly decreased the diameters of the seminiferous tubules compared to those of the scrotal testis by 82% in 7 days, 69% in 14 days, 71% in 21 days, and 45% in 30 days (P<0.01) (Figure 2B). After thirty days from induction of cryptorchidism, the operated testis was markedly smaller than the scrotal testis (Figure 3A), and sperm were not found in the cauda epididymis of cryptorchid testis (Figure 3B) compared with scrotal testis (Figure 3C).
To identify the changes of cell number in the canine testes after surgically induced cryptorchidism, immunohistochemistry was used. Expression of PGP9.5 was detected to identify undifferentiated spermatogonia, and expression of GATA-binding protein 4 was detected to recognize Sertoli cells in the scrotal and cryptorchid testes. PGP9.5-positive spermatogonia at days 7, 14, 21, and 30 after surgery are shown in Figure 4A. Abdominal testes exhibited significantly lower numbers of spermatogonia than did scrotal testes, (Figure 4B). On day 30 after inducing cryptorchidism, the numbers of spermatogonia in the abdominal testes reached the lowest level, averaging 2.6±0.89. This was significantly lower than the average numbers of 29.4±1.4 as measured in the control testes (Figure 4B). Sertoli cells were visualized using the GATA4 marker, and no significant difference was observed in the numbers of GATA4 positive cells in scrotal and cryptorchid testes 7,14, 21, and 30 days after surgically induced cryptorchidism (Figure 5).
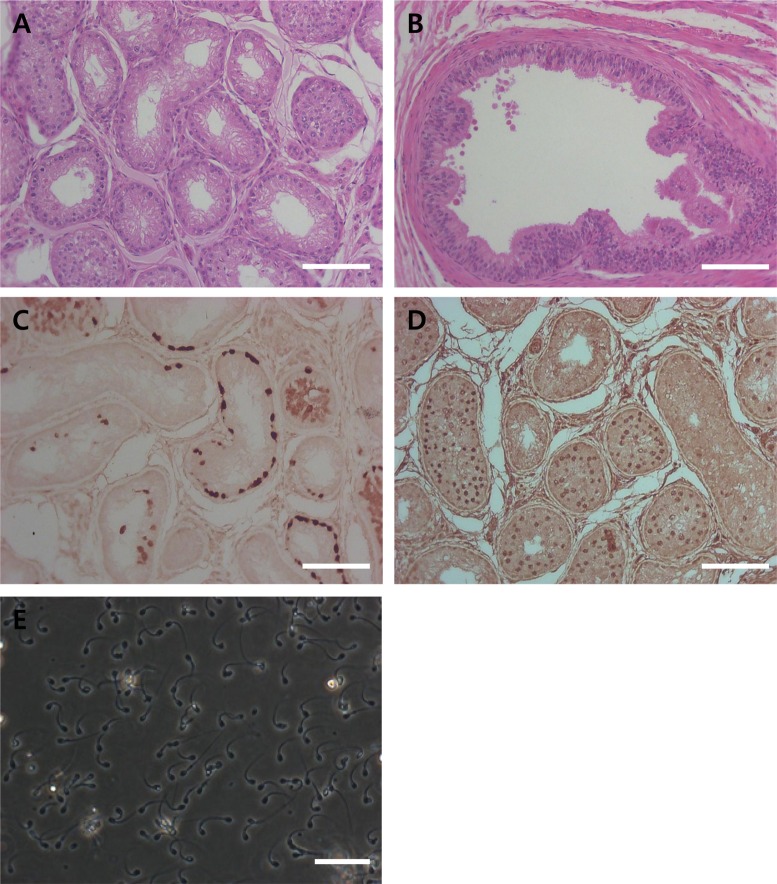
To confirm the regeneration of germ cells in cryptorchid testes after orchiopexy procedures, H & E staining and immunohistochemistry analysis were performed. Differentiated germ cells were detected in orchiopexy testes 4 weeks after orchiopexy (Figure 6A, D). However, sperm was not detected in orchiopexy testes and epididymis 4 weeks after orchiopexy (Figure 6A, B). Immunohistochemistry results showed PGP9.5 positive un-differentiated spermatogonia and SCP3 positive primary spermatocytes in seminiferous tubules of orchiopexy testes (Figure 6C, D). After eight weeks from orchiopexy, attempts at manual semen collection succeeded in all two dogs (Figure 6E).
Spermatogonial stem cell transplantation is a powerful technology to conserve endangered species and propagate the transgenic animals [18]. For preparation of the recipient testes in successful SSC transplantation, maximal depletion of endogenous germ cells with minimal effect on testicular microenvironment, are required [10]. Chemotherapeutic drugs, such as busulfan, have been used to prepare recipients for transplantation in several species. However, busulfan treatment in dog has not been tried to make recipient for SCC transplantation, and considerable variation between individual animals has been observed for any given dose level by intravenous administration [19]. Focal irradiation is another method used for canine recipient preparation, but all five recipient dogs had abnormal sexual behaviors such as no ejaculation and low libido in a previous study [3].
In the present study, we found that the surgical induction of abdominal cryptorchidism in mature dogs is a safe and feasible method to prepare recipients for SSC transplantation. It has been generally accepted that cryptorchidism leads to spermatogenic damage and that orchiopexy improves spermatogenesis. However, the extent of the damage caused by cryptorchidism and the degree of recovery caused by subsequent orchiopexy remain unclear. Our approach was based on three considerations: 1) Endogenous germ cells of recipient can be depleted by artificial induction of cryptorchidism to create the empty space for exogenous donor-derived SSCs; 2) Sertoli cells, supporter cells of SSCs, are not influenced by cryptorchidism; and 3) Orchiopexy can be lead to the return of normal spermatogenesis in cryptorchid testes.
Several studies showed a significant decrease in the diameter of seminiferous tubules in cryptorchid testis [2021]. Furthermore, in cryptorchid testis, testicular spermatogenesis markedly delayed, as evident by the significantly reduced diameter of seminiferous tubules [22]. Our data showed a remarkable decrease in the diameter of seminiferous tubules in cryptorchid testis compared to those of scrotal testis 7 days after surgery (Figure 2). Thirty days after the induction of cryptorchidism, we observed that the decrease in testicular size occurred simultaneously with histological changes in the germ cell population within the seminiferous tubules of cryptorchid testis (Figure 3, 4). Germ cell depletion resulted in a great number of empty spaces within the seminiferous tubules, which could facilitate the migration and location of transplanted SSCs from the seminiferous lumen to the basal compartments. These results were consistent with previously reported observations.
PGP9.5 is a deubiquitinating enzyme that controls mammalian gametogenesis in the testes [23]. In addition, PGP9.5 is expressed in undifferentiated spermatogonia of dogs testis [24]. The GATA4 transcription factor is implicated in the development and function of the mammalian testis [25]. During early testicular development, GATA4 is expressed in pre-sertoli cells, sertoli cells, fetal leydig cells, and other testicular somatic cells [26]. However, postnatal GATA4 expression is found mainly in sertoli cells and adult ledig cells [27]. Here, we observed that PGP9.5 expression in undifferentiated spermatogenic cells was significantly reduced in surgical cryptorchidism models, but the expression of GATA4 sertoli cells was not altered (Figure 4, 5). In the present study, we oberved no difference between the number of sertoli cells before and after the operation. Furthermore, this data indicates that induced high temperature specifically depleted germ cells in dog testes.
Orchiopexy is a surgery to move a cryptorchid testis to the scrotum and permanently fix it there to recover spermatogenesis. Jegou et al. [28] showed that cryporchidism surgically induced 14 day-old rats subsequently subjected to orchiopexy at day 35 gained full fertility. It is clear that early orchiopexy in experimentally crypotorchid animls prevents against testicular damage and infertility [29]. The results from the present study confirm that orchiopexy, before severe damage of endogeneous germ cells, can reverse the regeneration of and restore spermatogenesis. The synaptonemal complex protein 3 (SCP3) is a meiosis-specific structural protein that appears at axial and lateral elements of the synaptonemal complex [30]. In this study, spermatogenic cells were detected in orchiopexy testis and SCP3 was detected in seminiferous tubules (Figure 6). This suggests that germ6 cells were regenerated and precede spermatogenesis by orchiopexy. In adult dogs, full recovery from 30 days of surgically induced cryptorchidism was achieved eight weeks after orchiopexy was performed (Figure 6). In conclusion, we have developed and described surgically induced cryptorchidism protocols to specifically deplete endogenous germ cells. This study might facilitate the transplantation of canine spermatogonial stem cells and provide insight into the cellular mechanisms of spermatogenesis in canine testes.
Acknowledgments
This study was supported by grant (PJ011865) from the Cooperative Research Program for Agriculture Science & Technology Development, Rural Development Administration, Republic of Korea.
References
1. de Rooij DG, Grootegoed JA. Spermatogonial stem cells. Curr Opin Cell Biol. 1998; 10(6):694–701. PMID: 9914171.

2. Brinster RL, Zimmermann JW. Spermatogenesis following male germ-cell transplantation. Proc Natl Acad Sci U S A. 1994; 91(24):11298–11302. PMID: 7972053.

3. Kim Y, Turner D, Nelson J, Dobrinski I, McEntee M, Travis AJ. Production of donor-derived sperm after spermatogonial stem cell transplantation in the dog. Reproduction. 2008; 136(6):823–831. PMID: 18768666.

4. Kim Y, Selvaraj V, Dobrinski I, Lee H, McEntee MC, Travis AJ. Recipient preparation and mixed germ cell isolation for spermatogonial stem cell transplantation in domestic cats. J Androl. 2006; 27(2):248–256. PMID: 16304210.

5. Honaramooz A, Behboodi E, Blash S, Megee SO, Dobrinski I. Germ cell transplantation in goats. Mol Reprod Dev. 2003; 64(4):422–428. PMID: 12589654.

6. Izadyar F, Den Ouden K, Stout TA, Stout J, Coret J, Lankveld DP, Spoormakers TJ, Colenbrander B, Oldenbroek JK, Van der Ploeg KD, Woelders H, Kal HB, De Rooij DG. Autologous and homologous transplantation of bovine spermatogonial stem cells. Reproduction. 2003; 126(6):765–774. PMID: 14748695.

7. Honaramooz A, Megee SO, Dobrinski I. Germ cell transplantation in pigs. Biol Reprod. 2002; 66(1):21–28. PMID: 11751259.
8. Brinster RL. Germline stem cell transplantation and transgenesis. Science. 2002; 296(5576):2174–2176. PMID: 12077400.

9. Ogawa T, Aréchaga JM, Avarbock MR, Brinster RL. Transplantation of testis germinal cells into mouse seminiferous tubules. Int J Dev Biol. 1997; 41(1):111–122. PMID: 9074943.
10. Brinster CJ, Ryu BY, Avarbock MR, Karagenc L, Brinster RL, Orwig KE. Restoration of fertility by germ cell transplantation requires effective recipient preparation. Biol Reprod. 2003; 69(2):412–420. PMID: 12672656.
11. Ogawa T, Dobrinski I, Brinster RL. Recipient preparation is critical for spermatogonial transplantation in the rat. Tissue Cell. 1999; 31(5):461–472. PMID: 10612257.

12. Honaramooz A, Behboodi E, Hausler CL, Blash S, Ayres S, Azuma C, Echelard Y, Dobrinski I. Depletion of endogenous germ cells in male pigs and goats in preparation for germ cell transplantation. J Androl. 2005; 26(6):698–705. PMID: 16291964.

13. Jahnukainen K, Ehmcke J, Quader MA, Saiful Huq M, Epperly MW, Hergenrother S, Nurmio M, Schlatt S. Testicular recovery after irradiation differs in prepubertal and pubertal non-human primates, and can be enhanced by autologous germ cell transplantation. Hum Reprod. 2011; 26(8):1945–1954. PMID: 21613315.

14. Zhang Z, Shao S, Meistrich ML. Irradiated mouse testes efficiently support spermatogenesis derived from donor germ cells of mice and rats. J Androl. 2006; 27(3):365–375. PMID: 16339450.

15. Griffiths J. The Structural Changes in the Testicle of the Dog when it is Replaced within the Abdominal Cavity. J Anat Physiol. 1893; 27(Pt 4):482–500.
16. Shirai M, Matsushita S, Kagayama M, Ichijo S, Takeuchi M. Histological changes of the scrotal testis in unilateral cryptorchidism. Tohoku J Exp Med. 1966; 90(4):363–373. PMID: 4382089.

17. Hall PF, Kew D, Mita M. The influence of temperature on the functions of cultured Sertoli cells. Endocrinology. 1985; 116(5):1926–1932. PMID: 2985365.

18. Hill JR, Dobrinski I. Male germ cell transplantation in livestock. Reprod Fertil Dev. 2006; 18(1-2):13–18. PMID: 16478598.

19. Deeg HJ, Schuler US, Shulman H, Ehrsam M, Renner U, Yu C, Storb R, Ehninger G. Myeloablation by intravenous busulfan and hematopoietic reconstitution with autologous marrow in a canine model. Biol Blood Marrow Transplant. 1999; 5(5):316–321. PMID: 10534062.

20. Monet-Kuntz C, Barenton B, Locatelli A, Fontaine I, Perreau C, Hochereau-de Reviers MT. Effects of experimental cryptorchidism and subsequent orchidopexy on seminiferous tubule functions in the lamb. J Androl. 1987; 8(3):148–154. PMID: 2886484.
21. Amat P, Paniagua R, Montero J. Seminiferous tubule degeneration in human cryptorchid testes. J Androl. 1985; 6(1):1–9. PMID: 2857707.

22. AbouZeid AA, Mousa MH, Soliman HA, Hamza AF, Hay SA. Intra-abdominal testis: histological alterations and significance of biopsy. J Urol. 2011; 185(1):269–274. PMID: 21075394.

23. Sutovsky P. Ubiquitin-dependent proteolysis in mammalian spermatogenesis, fertilization, and sperm quality control: killing three birds with one stone. Microsc Res Tech. 2003; 61(1):88–102. PMID: 12672125.

24. Harkey MA, Asano A, Zoulas ME, Torok-Storb B, Nagashima J, Travis A. Isolation, genetic manipulation, and transplantation of canine spermatogonial stem cells: progress toward transgenesis through the male germ-line. Reproduction. 2013; 146(1):75–90. PMID: 23690628.

25. Viger RS, Guittot SM, Anttonen M, Wilson DB, Heikinheimo M. Role of the GATA family of transcription factors in endocrine development, function, and disease. Mol Endocrinol. 2008; 22(4):781–798. PMID: 18174356.

26. Bielinska M, Seehra A, Toppari J, Heikinheimo M, Wilson DB. GATA-4 is required for sex steroidogenic cell development in the fetal mouse. Dev Dyn. 2007; 236(1):203–213. PMID: 17096405.

27. Ketola I, Rahman N, Toppari J, Bielinska M, Porter-Tinge SB, Tapanainen JS, Huhtaniemi IT, Wilson DB, Heikinheimo M. Expression and regulation of transcription factors GATA-4 and GATA-6 in developing mouse testis. Endocrinology. 1999; 140(3):1470–1480. PMID: 10067876.
28. Jegou B, Peake RA, Irby DC, de Kretser DM. Effects of the induction of experimental cryptorchidism and subsequent orchidopexy on testicular function in immature rats. Biol Reprod. 1984; 30(1):179–187. PMID: 6141812.
29. Karpe B, Plöen L, Hagenäs L, Ritzén EM. Recovery of testicular functions after surgical treatment of experimental cryptorchidism in the rat. Int J Androl. 1981; 4(2):145–160. PMID: 6114042.

30. Parra MT, Viera A, Gómez R, Page J, Benavente R, Santos JL, Rufas JS, Suja JA. Involvement of the cohesin Rad21 and SCP3 in monopolar attachment of sister kinetochores during mouse meiosis I. J Cell Sci. 2004; 117(Pt 7):1221–1234. PMID: 14970259.

Figure 1
Surgically induced cryptorchidism and orchiopexy procedures. (A) The testis was pushed out through the paramedial incision. The arrow indicates the inguinal ring. (B) The testicle was introduced into the abdominal cavity to induce experimental cryptorchidism. (C) The inguinal ring was sutured to prevent the normal descent of the testicle. (D) The cryptorchid testicle was moved into the scrotum and permanently fixed there.
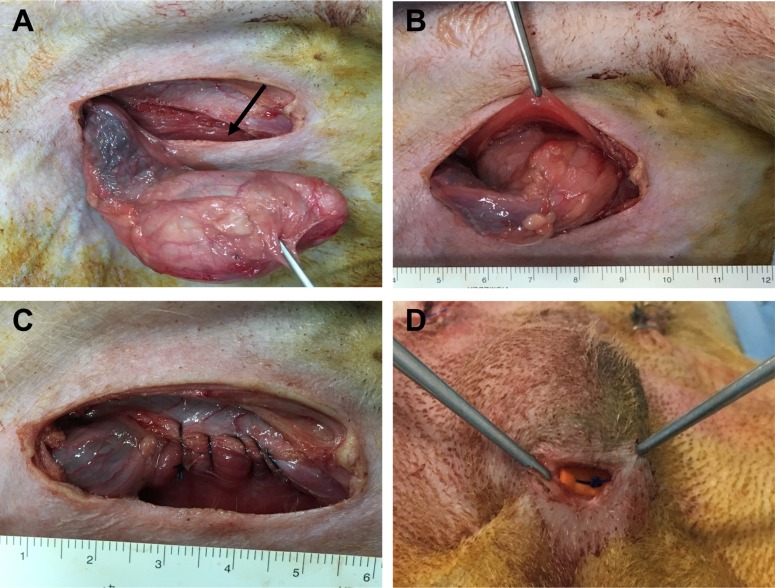
Figure 2
Morphological changes in scrotal (control) and surgically induced cryptorchidism in dog testes. (A) Hematoxylin and eosin staining results at 7, 14, 21, and 30 days after surgery (D7, D14, D21 and D30). (B) Comparison of seminiferous tubule diameter of dog testes in control and experimental groups. Data are presented as mean±SD. ***P<0.01, compared with the control. The scale bars indicate 100 mm.
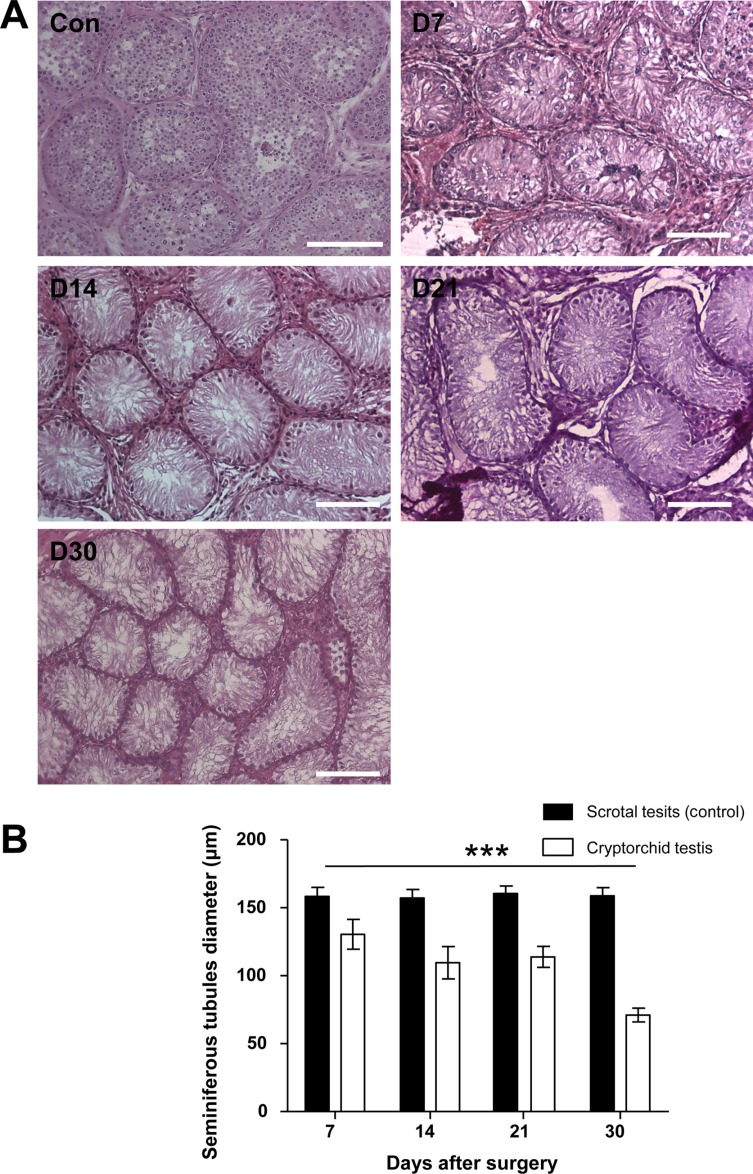
Figure 3
Macroscopic and histological comparison of control dog testes and testes subjected to artificial cryptorchidism for 30 days. (A) Comparison of testis size in cryptorchidism and scrotal testis on day 30 after surgery. Representative section of the cauda epididymis in cryptorchid (B) and scrotal (C) testis. C (Left) is a cryptorchid testis and S (Right) is a scrotal testis. Arrow indicates sperm. The scale bars indicate 100 mm.
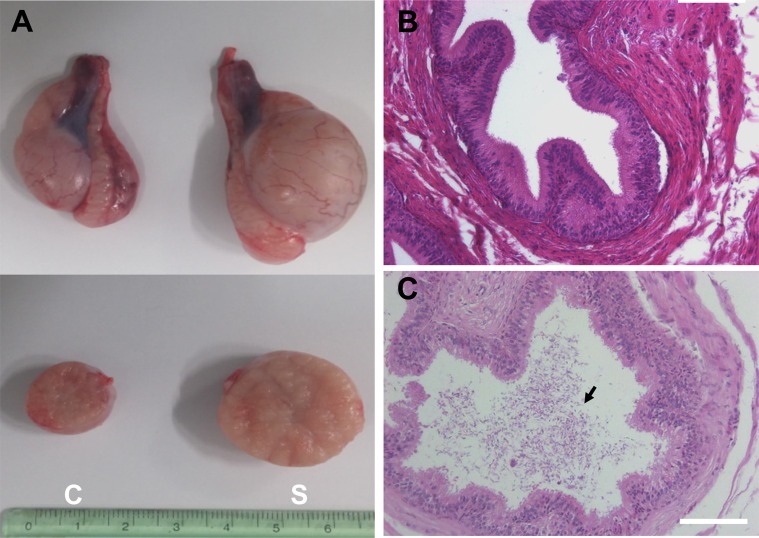
Figure 4
Canine spermatogonia as detected by PGP9.5 immunohistochemisty staining. (A) Positive staining for anti-PGP9.5 antibody 7, 14, 21, and 30 days after surgery (D7, D14, D21, and D30). (B) Comparison of positive cell numbers in control and cryptorchidism groups on different days. Arrows indicate PGP9.5 positive cells. Results are expressed as the mean±SD. P<0.05 was considered statistically significant. The scale bars indicate 100 mm.
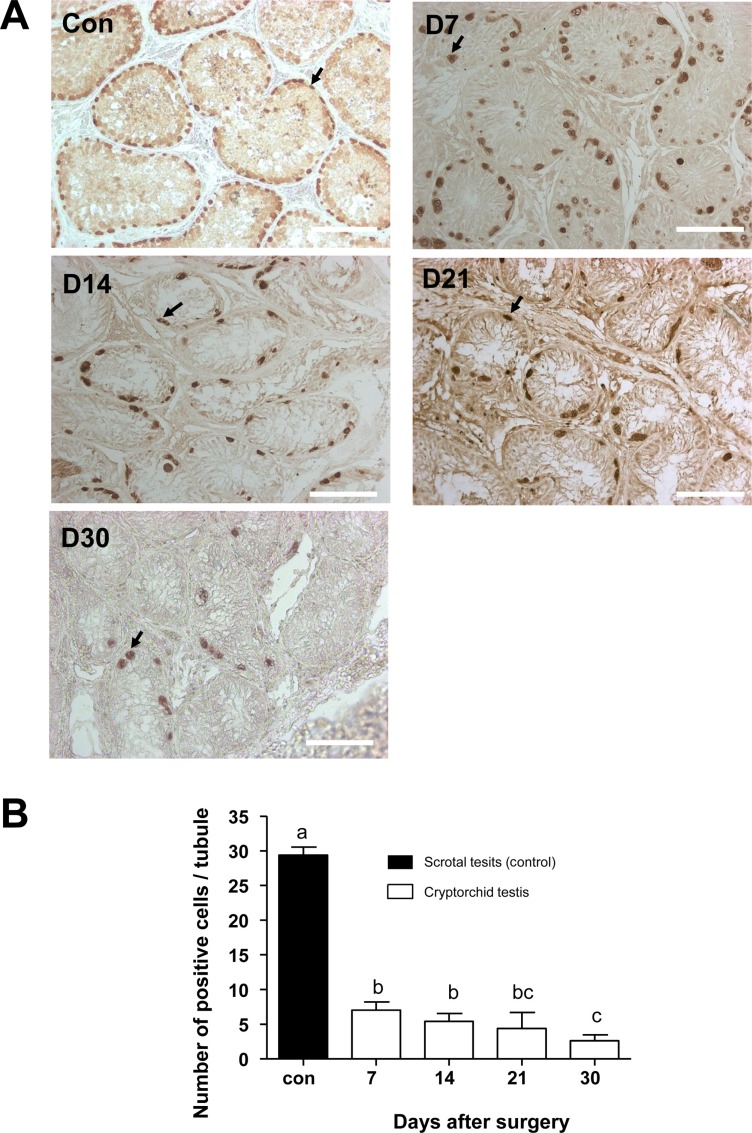
Figure 5
Proportion of canine Sertoli cells as detected by GATA4 immunohistochemical staining. (A) Positive staining of GATA4 antibody 7, 14, 21, and 30 days after surgery (D7, D14, D21, and D30). Arrows indicate GATA4 positive cells. (B) Comparison of positive cell numbers in the control cryptorchidism groups on different days. Results are expressed as the mean±SD. P<0.05 was considered statistically significant. The scale bars indicate 100 mm.
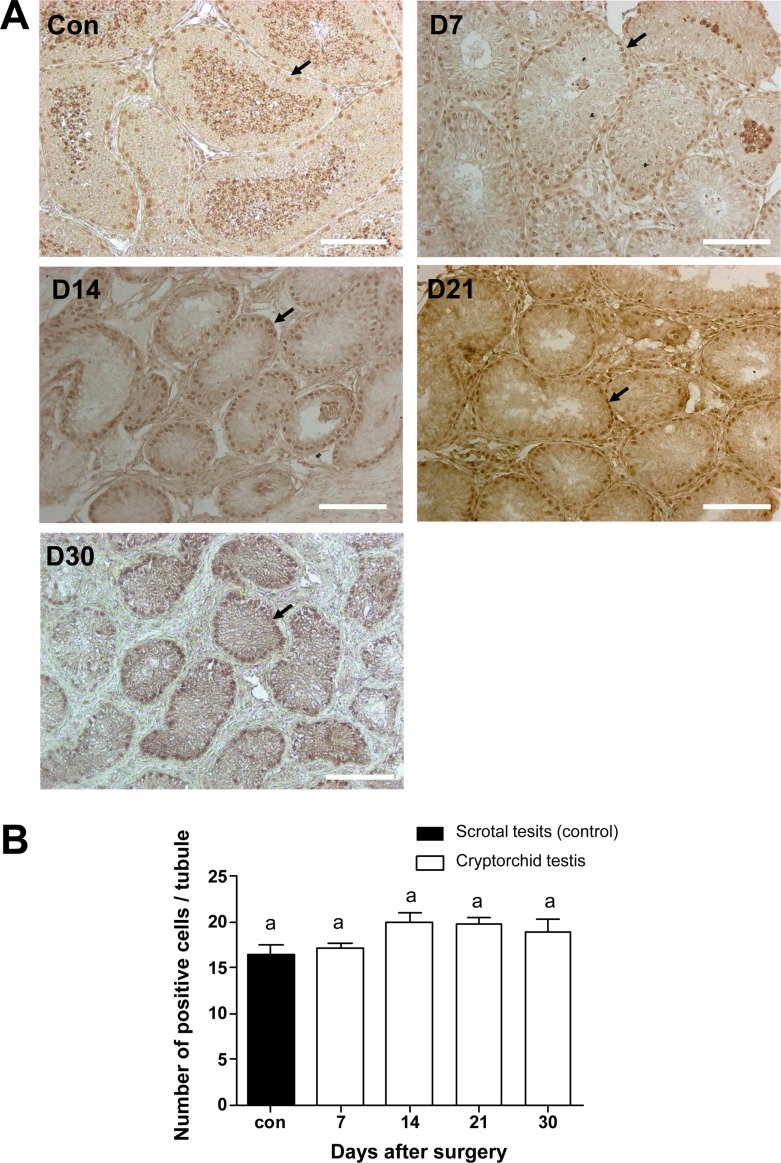
Figure 6
Histological and immunohistochemical analyses of testis and epididymis tissue from testis after orchiopexy (A, B, C and D). (A) H & E staining of testis and (B) epididymis. (C) PGP9.5 and (D) SCP3 staining of testis 4 weeks after orchiopexy. (E) Ejaculated sperm 8 weeks after orchiopexy. The scale bars indicate 100 mm.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download