INTRODUCTION
Table 1
Acute and delayed tick bite effects in Australia

| Acute effects | |||
| Localised reactions | Small local reaction | Papules (small bumps) may form at the site of attachment within 1 to 3 days, not thought to be allergic response [11]. | |
| Large local reaction | Localised erythema and oedema greater than 5-cm diameter, commencing 4 hours postbite and lasting up to 10 days [12]. Typically self-limiting but IgE-mediated so can indicate hypersensitivity and therefore increased risk of tick anaphylaxis [13]. | ||
| Systemic allergic reactions | Allergic reaction (mucocutaneous) | Urticaria, erythema/flushing and/or angioedema distant from bite site, typically shortly after disturbing or attempting to remove a tick [1314]. | |
| Anaphylaxis | Typical skin features plus involvement of respiratory, cardiovascular and/or gastrointestinal symptoms, typically shortly after disturbing or attempting to remove a tick [1314]. | ||
| Delayed effects | |||
| Paralysis | Ascending flaccid paralysis | Holocyclotoxins cause inhibition of acetylcholine release at the neuromuscular junction [15]. Symptoms become apparent from day 3 but peak on day 4 or 5, may be acutely worsened by removal and progress for up to 48 hours after removal [16]. | |
| Systemic allergic reaction | Mammalian meat allergy | First described by van Nunen et al. (2007) [23], cross-reactive hypersensitivity to the carbohydrate moiety alpha-gal, likely picked up from a previous feed on a bandicoot or other mammal, causes anaphylactic reaction 3–6 hours after consuming mammalian meat [18]. | |
| Infective | Tick-borne disease | Queensland Tick Typhus (Rickettsia australis) [19] | |
| Q fever (Coxiella burnetti) [19] | |||
| Australian Spotted Fever (Rickettsia honei subsp. Marmionii) [19] | |||
| Flinders Island Spotted Fever (Rickettsia honei) [19] | |||
| Lyme disease (Borrelia burgdorferi) [19] – remains controversial in Australia with no proven vector | |||
| Cellulitis | Opportunistic infection through skin break [20], e.g., staphylococcal | ||
| Retained mouthparts | Granuloma | Granulation tissue which forms around foreign body [21] | |
| Abscess | Foreign body reaction combined with opportunistic pathogen [21] | ||
| Autoimmune disease | Graves' disease | Only one documented case published in the literature to date [22] | |
(1) IH has a geographical distribution along the eastern starboard of Australia from as far north as Cape Tribulation to as far south as ACT and Victoria, matching the distribution of a large proportion of Australia's human population [1].
(2) IH commonly bites humans as well as other mammals although its main host has been thought to be the bandicoot [1].
(3) IH is capable of the most severe effects on humans – anaphylaxis and paralysis
(4) IH has also been implicated in causing mammalian meat allergy as well as transmitting the tick-borne diseases Q fever and Australian Tick Typhus [232425].
MATERIALS AND METHODS
(1) Nymphs/Larvae: careful dab of Lyclear Scabies Cream, covering the whole tick
(2) Adult ticks: five sprays of Wart-Off Freeze, held 1 cm above the tick
(1) Any acute onset illness with typical skin features (urticaria, erythema/flushing, and/or angioedema) PLUS involvement of respiratory, cardiovascular and/or gastrointestinal systems
(2) Any acute onset of hypotension or bronchospasm or upper airway obstruction where anaphylaxis is considered possible, even if typical skin features are not present
RESULTS
Fig. 1
Inclusion and exclusion criteria flowchart. MVH, Mona Vale Hospital; ED, Emergency Department; eMR, electronic medical record.
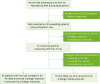
Table 2
Removal methods of 50 patients presenting after tick removal (allergic reaction/total)

Table 3
Removal methods of 71 patients presenting with ticks in situ (allergic reaction/total)

Table 4
Allergic reactions in 71 patients presenting with ticks in situ





 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download