Abstract
Alpha-fetoprotein-Producing gastric cancer is associated with poor prognosis because of frequent liver and lymph node metastasis. We present a case with synchronous liver metastasis who survived for 5 years. A 69-year-old man with upper abdominal pain was referred to our hospital. Gastrointestinal endoscopy revealed a Borrmann II-like tumor in the lower part of the stomach. Computed tomography revealed a tumor in the left lobe of the liver. Serum alpha-fetoprotein levels were markedly increased. We performed distal gastrectomy after administering oral tegafur/gimeracil/oteracil potassium and administered hepatic intra-arterial cisplatin injection. Liver metastasis showed partial response on computed tomography. Despite left hepatic lobectomy, further metastases to the liver and mediastinal lymph nodes became difficult to control. After sorafenib tosylate administration, stabilization of the disease was observed for 4 months. We conclude that hepatic intra-arterial chemotherapy and oral administration of sorafenib tosylate may potentially improve the prognosis in such cases.
Alpha-fetoprotein-producing gastric cancer (AFPGC) is a rare subtype of gastric cancer associated with a poor prognosis because it readily metastasizes to the liver and lymph nodes. Despite attempts to treat AFPGC using many types of therapies, the prognosis remains bleak. Here we report the case of a patient with AFPGC treated using multimodal therapy who survived for 5 years. In particular, we focus on the effectiveness of hepatic intra-arterial (HIA) chemotherapy combined with oral administration of sorafenib tosylate (sorafenib).
A 69-year-old man with upper abdominal pain was referred to our hospital in August 2006 by a local physician. Upper gastrointestinal endoscopy revealed a Borrmann type II-like tumor in the posterior wall of the lower part of the stomach (Fig. 1A). The biopsy specimen revealed well-differentiated adenocarcinoma, and immunohistological staining showed that tumor cells were positive for alpha-fetoprotein-producing (AFP). An upper gastrointestinal series radiography showed an elevated lesion with a central depression (Fig. 1B) in the lower part of the stomach. Laboratory examination revealed mildly elevated levels of aspartate aminotransferase (AST 67 IU/L), lactate dehydrogenase (834 IU/L), and alkaline phosphatase (565 IU/L). Serum level of carcinoembryonic antigen and CA19-9 was normal, but that of AFP was markedly increased (160,000 ng/ml).
Abdominal contrast-enhanced computed tomography (CECT) revealed a 14 cm long hypovascular tumor in the left lobe of the liver (Fig. 1C). On the basis of these findings, we diagnosed AFPGC with synchronous liver metastasis.
Our initial treatment plan was to perform a distal gastrectomy and an extended left lobectomy of the liver. However, another liver metastasis was found in the right lobe of the liver by the intraoperative sonography. We limited the surgery to distal gastrectomy with D2 lymph node dissection and Billroth I reconstruction in August 2006. The resected specimen was determined to be a 2.8 cm long Borrmann type II-like tumor (Fig. 1D).
Histopathological examination of the gastric tumor revealed a well-differentiated adenocarcinoma with neoplastic growth of hepatocyte-like cells (Fig. 2A). The level of invasion was submucosal and 3 of 35 dissected lymph nodes were positive for metastasis. Intraoperative biopsy of the liver tumor showed features similar to those of the gastric tumor (Fig. 2B). Immunohistological staining showed that tumor cells in both the gastric tumor (Fig. 2C) and the biopsy specimen from the liver tumor (Fig. 2D) were positive for AFP.
After surgery, we placed a catheter into the proper hepatic artery for HIA. Chemotherapy using oral tegafur/gimeracil/oteracil potassium (TS-1, Taiho Co. Ltd., Tokyo, Japan) (120 mg/body/day from days 1 to 21)/HIA-cisplatin (CDDP) (30 mg/body/day on days 8 and 21, every 28 days) was started at day 15 after the surgery. After 4 courses, CECT revealed that the size of the metastatic tumor in the left lobe had decreased to 53.6% of the peak (Fig. 3A), and the small liver metastasis had disappeared. The serum AFP level had decreased to 2,910 ng/ml.
Six months after the surgery, the serum level of AFP began to increase, that is why we performed a left lobectomy. The resected specimen showed atrophic change in the left lobe and an odd-shaped soft liver tumor (Fig. 3B). On cut sections, the tumor was yellowish and highly denatured (Fig. 3C). Microscopic findings revealed degenerative changes and necrosis in almost all sections of the tumor. Viable cells were similar in structure to the microscopic findings of the primary gastric cancer. After liver resection, 9 courses of adjuvant oral TS-1/HIA-CDDP and oral TS-1/CPT-11 by drip infusion of vein (div) were administered.
Twenty-five months after the first surgery, we performed a partial lung resection because of new metastasis to the lung (Fig. 3D). Four months after partial lung resection, radiotherapy was performed to treat 2 new lung metastases. We obtained complete response transiently, but another new lung metastasis and para-aortic lymph node metastases appeared immediately. To treat these metastatic lesions, we performed chemotherapy sequentially as follows: 6 courses of paclitaxel (PTX) (div); 2 courses of 5'-Doxifluridine (DFUR) (oral)/Docetaxel (DOC) (div)/CDDP (div); 5 courses of DFUR (oral)/DOC (div); 8 courses of intravenous injection of 5-fluorouracil (5FU)/Adriamycin/Mitomycin-C (FAM); 2 courses of TS-1 (oral)/CPT-11 (div); and 6 courses of PTX (div) (Fig. 4).
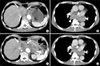
In spite of these combination chemotherapies, metastatic lesions became difficult to control at 54 months after the first surgery, and the serum AFP level rose to 84,434 ng/ml. Liver metastases (Fig. 5A) and mediastinal lymph node metastasis enlarged gradually (Fig. 5B). The patient complained of respiratory distress resulting from bronchial oppression by enlarged lymph nodes. We could not use trastuzumab because immunohistochemical analysis revealed that human epidermal growth factor receptor type 2 was not expressed in either the primary lesion or the metastatic liver tumor. We proposed best supportive care, but the patient wanted to try a molecular-targeted drug. The administration of oral sorafenib tosylate (sorafenib) was begun at a dose of 800 mg/body/day using of a private import. After 2 months, his serum level of AFP dropped from 100,000 to 59,198 ng/ml. CECT revealed marked attenuation of vascularity at the liver metastasis, but a decrease in tumor size was not observed (Fig. 5C). The mediastinal lymph nodes shrunk in size by 16.2% (Fig. 5D). The patient's respiratory distress was completely alleviated, and stable disease was observed for a period of 4 months. The only adverse events observed were grade 1 hoarseness, increased AST, and hand-foot syndrome. The patient eventually died from cancer cachexia at 60 months after the first surgery.
AFPGC is a rare tumor, accounting for only 1.3~15% of all gastric cancers.1 Previous reports suggest that the poor prognosis of AFPGC relative to non-AFPGC is because of its biological aggressiveness, as evidenced by its higher frequency for liver and lymph node metastasis.1-3 AFPGC with liver metastasis has a particularly dismal prognosis, regardless of whether it is synchronous or metachronous. Previous studies showed that the 5-year survival rate and median survival times were 0~3.8% and 9~11.4 months, respectively.1,4
On the other hand, several recent studies have demonstrated that AFPGC is highly chemosensitive compared with non-AFPGC. Kochi et al.5 reported the effectiveness of systemic 5FU/leucovorin/etoposide/CDDP (FLEP) combination therapy. After treating 10 patients with AFPGC and 47 with non-AFPGC using the FLEP regimen, they concluded that the AFPGC patients had a significantly better response rate and better disease-free and overall survival. Inoue et al.6 reported that 4 AFPGC patients with liver metastasis survived for 5 years through multimodal therapy and concluded the prognosis of AFPGC is not as poor as previously thought. These studies encourage us to not give up when treating AFPGC patients. However, to the best of our knowledge, only 10 cases have been reported in which the patient achieved 5-year survival when liver metastasis were present.6-8
In our case, metastasis was initially limited to the liver, so we selected HIA-CDDP combination chemotherapy with the objective of increasing the level of CDDP inside the liver tumor. In addition to the direct response against the large liver metastasis, we thought this drug combination might prevent further liver metastasis. Three AFPGC patients treated with HIA were previously reported to have survived over 5 years. Sakurai et al.9 administered oral tegafur/uracil (UFT)/HAI-FAM, and the patient survived for 6 years. The administration of HAI-CDDP/MMC by Kajikawa et al. resulted in the 6-year survival of the patient. Kobayashi et al. administered UFT/HIA-MMC orally, achieving an 11-year survival.7 Several reports suggest that even when long-term survival is not achieved, HIA-treatment might improve the prognosis over that of systemic chemotherapies.10,11 We think that a regimen containing HAI is worth trying if metastasis is limited to the liver.
A significant finding brought forth by this case is the effectiveness of sorafenib in treating metastatic AFPGC lesions. Sorafenib is a multi-kinase-targeting oral drug that inhibits intercellular signaling pathways and extracellular receptors, including vascular endothelial growth factor receptor and platelet-derived growth factor receptor.12 In patients with advanced hepatocellular carcinoma (HCC), randomized studies revealed that sorafenib prolonged the median survival time by nearly 3 months.12 Recently, Kim et al.13 showed the effectiveness of sorafenib in combination with CPT-11/CDDP systemic chemotherapy as a first line therapy for advanced gastric cancer. They reported the response rate was moderately high (62.5%, 10 of 16 patients), but the median overall survival was <1 year.
Several studies have reported that the hepatocyte growth factor (HGF)/c-Met pathway is activated in both AFPGC and HCC.14,15 Activated HGF/c-Met has been observed to enhance downstream signaling cascades such as the JAK/STAT3 and Raf/MEK/ERK pathways, resulting in cell proliferation.15 One study reported that sorafenib inhibits these pathways and suppresses the progression of HCC.12 Sorafenib has been speculated to inhibit the activity of the Raf/MEK/ERK and JAK/STAT3 pathways in AFPGC as in HCC. Using CECT, we found that the vascularity of the tumors decreased markedly during sorafenib treatment. We hypothesize that the inhibition of VERFR and PDGFR by sorafenib led to this observed decrease in tumor vascularity. This report is the first to suggest the possible use of sorafenib for treating AFPGC. Further studies and clinical trials are required to confirm the efficacy of sorafenib in the treatment of AFPGC.
Figures and Tables
Fig. 1
(A) Upper gastrointestinal endoscopy revealing a Borrmann type II like tumor at the posterior wall of the lower part of the stomach. (B) Upper gastrointestinal series showing the elevated lesion with central depression (arrow). (C) Abdominal CECT revealing a 14-cm-long hypovascular tumor the left lobe of the liver. (D) The resected specimen showing a Borrmann type II 2.8-cm-long tumor at the lower part of the stomach (arrow). CECT = contrast-enhanced computed tomography.
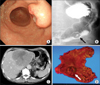
Fig. 2
(A) Histopathological examination revealing well-differentiated adenocarcinoma with neoplastic growth of hepatocyte-like cells in the gastric tumor (H&E stain, ×100). (B) The biopsy specimen from the liver tumor is similar to gastric tumor (H&E stain, ×100). (C) Immunohistological staining showing positive result for AFP in the gastric tumor (AFP, ×100). (D) The biopsy specimen from the liver tumor was also positive for AFP staining (AFP, ×100). AFP = alpha-fetoprotein.
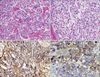
Fig. 3
(A) CECT showing the liver metastasis in the left lobe decreased to 53.6% after 4 courses of oral TS-1/hepatic intra-arterial injection of cisplatin (arrows). (B) Resected specimen showing atrophic changes in the left lobe and a soft, odd-shaped liver tumor. (C) On cut section, the liver tumor is shown to be yellowish and highly-denatured inside of the tumor (arrows). (D) CECT showing the lung tumor at the left lobe of the lung (arrows). CECT = contrast-enhanced computed tomography.
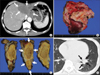
Fig. 4
Patient's therapeutic course. AFP = alpha-fetoprotein; TS-1 = tegafur/gimeracil/oteracil potassium; HAI-CDDP = hepatic artery injection; CDDP = cisplatin; PTX = paclitaxel; DFUR = 5'-Doxifluridine; DOC = Docetaxel; FAM = 5-fluorouracil/Adriamycin/Mitomycin-C.

Fig. 5
(A) CECT after 54 months from first operation revealing the increasing size of liver metastases (arrows). (B) CECT revealing the increasing size of the mediastinal lymph nodes metastases (arrowheads). (C) CECT after 2 months of administering sorafenib revealing marked attenuation of vascularity at the liver metastasis (arrows). (D) The mediastinal lymph nodes shrunk by 16.2% in size (arrowheads). CECT = contrast-enhanced computed tomography.
Acknowledgments
The authors would like to thank Enago (www.enago.jp) for the English language review.
References
1. Chun H, Kwon SJ. Clinicopathological characteristics of alpha-fetoprotein-producing gastric cancer. J Gastric Cancer. 2011. 11:23–30.

2. Kono K, Amemiya H, Sekikawa T, Iizuka H, Takahashi A, Fujii H, et al. Clinicopathologic features of gastric cancers producing alpha-fetoprotein. Dig Surg. 2002. 19:359–365.

3. Ishigami S, Natsugoe S, Nakashima H, Tokuda K, Nakajo A, Okumura H, et al. Biological aggressiveness of alpha-fetoprotein (AFP)-positive gastric cancer. Hepatogastroenterology. 2006. 53:338–341.
4. Adachi Y, Tsuchihashi J, Shiraishi N, Yasuda K, Etoh T, Kitano S. AFP-producing gastric carcinoma: multivariate analysis of prognostic factors in 270 patients. Oncology. 2003. 65:95–101.

5. Kochi M, Fujii M, Kaiga T, Takahashi T, Morishita Y, Kobayashi M, et al. FLEP chemotherapy for alpha-fetoprotein-producing gastric cancer. Oncology. 2004. 66:445–449.

6. Inoue M, Sano T, Kuchiba A, Taniguchi H, Fukagawa T, Katai H. Long-term results of gastrectomy for alpha-fetoprotein-producing gastric cancer. Br J Surg. 2010. 97:1056–1061.

7. Asami T, Kokawa A, Sugimori K, Tomita N, Shirato K, Morimoto M, et al. A case of AFP-producing gastric cancer with multiple liver metastases responding to CPT-11 and cisplatin combination chemotherapy. Gan To Kagaku Ryoho. 2002. 29:1985–1988.
8. Yoshioka M, Inoue N, Someda H, Noguchi M, Sawada M, Azuma K, et al. A case of AFP-producing gastric cancer resected after efficient S-1/CDDP combination chemotherapy. Gan To Kagaku Ryoho. 2011. 38:105–108.
9. Sakurai N, Yamauchi J, Fukushima N, Shibuma H, Ikeda E, Sasou S. A case of non-recurred long-term survival after chemotherapy for liver metastasis of AFP-producing gastric carcinoma. Jpn J Gastroenterol Surg. 2005. 38:418–423.

10. Nagahama T, Maruyama M, Toukairin Y, Baba H, Yoshida T, Kure N, et al. Hepatic arterial injection therapy (HAI) for metastatic liver tumor from gastric cancer. Gan To Kagaku Ryoho. 2000. 27:1920–1923.
11. Takada J, Kenno S, Aoki T, Hamada H, Katsuki Y. A case in which intra-arterial chemotherapy for simultaneous hepatic metastases markedly improved AFP-producing gastric cancer. Gan To Kagaku Ryoho. 2009. 36:2326–2329.
12. Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. SHARP Investigators Study Group. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008. 359:378–390.

13. Kim C, Lee JL, Choi YH, Kang BW, Ryu MH, Chang HM, et al. Phase I dose-finding study of sorafenib in combination with capecitabine and cisplatin as a first-line treatment in patients with advanced gastric cancer. Invest New Drugs. 2012. 30:306–315.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download