Abstract
Purpose
Endothelial dysfunction and peripheral neuropathy are important mechanisms responsible for diabetes-induced erectile dysfunction (ED). Nerve injury-induced protein 1 (Ninjurin 1) is known to be related to neuroinflammatory processes and is also reported to induce vascular regression during the developmental period. In the present study, we determined the differential expression of Ninjurin 1 in penile tissue of streptozotocin (STZ)-induced diabetic mice with ED.
Materials and Methods
Diabetes was induced in 8-week-old C57BL/6J mice by intraperitoneal injections of STZ (50 mg/kg for 5 days). Eight weeks later, erectile function was measured by electrical stimulation of the cavernous nerve (n=6 per group). The penis was then harvested for immunohistochemical analysis and Western blot analysis for Ninjurin 1 (n=4 per group). We also determined Ninjurin 1 expression in primary cultured mouse cavernous endothelial cells (MCECs) incubated under the following conditions: normal glucose condition (5 mM), high-glucose condition (30 mM), and high-glucose condition (30 mM)+insulin (1 nM).
Results
The expression of Ninjurin 1 protein was significantly higher in both cavernous endothelial cells and the dorsal nerve bundle of diabetic mice than in those of controls. In the in vitro study in MCECs, Ninjurin 1 expression was also significantly increased by the high-glucose condition and was returned to baseline levels by treatment with insulin.
Diabetes mellitus is a major cause of erectile dysfunction (ED), and the prevalence of ED is more common and the symptoms of ED occur earlier in diabetic men than in the general population [1,2]. The etiology of ED in diabetes is multifactorial. The suggested mechanisms for diabetic ED include damage to the cavernous endothelial and smooth muscle cells at both structural and functional levels, peripheral autonomic neuropathy, and endocrine dysfunction, such as hypogonadism [3-7]. It is well known that men with diabetic ED respond poorly to the currently available oral phosphodiesterase-5 (PDE5) inhibitors compared with nondiabetic men [8,9]. The reduced responsiveness to the PDE5 inhibitors in men with diabetes may be related to the severity of functional and structural derangements in the corporal vasculature as well as the degree of peripheral neuropathy [6,10,11].
Nerve injury-induced protein 1, Ninjurin 1, is a cell surface protein related to peripheral nerve injury that is known to be profoundly up-regulated in the distal segment and proximal neuroma of the transected sciatic nerve [12]. It was also reported that Ninjurin 1 is responsible for the initiation and progression of multiple sclerosis [13], an autoimmune inflammatory disease of the central nervous system (CNS) characterized by demyelination and axonal damage. Moreover, Ninjurin 1 plays an important role in blood vessel homeostasis during the embryonic period. A recent study reported that Ninjurin 1 resulted in the regression of the hyaloids vascular system, a capillary vascular network responsible for the growth and maturation of the lens during early ocular development, through induction of angiopoietin-2 (Ang2), an endogenous antagonist of angiopoietin-1 (Ang1) involved in destabilization of the blood vessels and endothelial cell apoptosis, and of Wnt7b, a critical mediator of cell death [14]. Inhibition of Ninjurin 1 by systemic administration of neutralizing antibody delays regression of the hyaloids vascular system [14]. These findings suggest the critical function of Ninjurin 1 in both neuropathy and blood vessel regression. Therefore, it is meaningful to examine the expression of Ninjurin 1 in diabetic ED, a disease characterized by neuropathy and vasculopathy.
Thus, in the present study, we determined the differential expression of Ninjurin 1 in both penile tissue of streptozotocin (STZ)-induced diabetic mice with ED in vivo and in primary cultured mouse cavernous endothelial cells (MCECs) in vitro.
Specific-pathogen-free C57BL/6J mice were purchased from Orient Bio (Seongnam, Korea). The experiments performed were approved by the Institutional Animal Care and Use Subcommittee of our university. Diabetes was induced in 8-week-old C57BL/6J mice by intraperitoneal injections of STZ (50 mg/kg) for 5 days consecutively as previously described [5]. At 8 weeks after the induction of diabetes, the diabetic animals and their age-matched controls were used for Western blot analysis, histologic examination, and physiologic erection study. Fasting and postprandial blood glucose levels were determined with an Accu-check blood glucose meter (Roche Diagnostics, Mannheim, Germany) before the mice were killed.
The mice from the control and diabetic groups were anesthetized with ketamine (100 mg/kg) and xylazine (5 mg/kg) intramuscularly. Bipolar platinum wire electrodes were placed around the cavernous nerve. Stimulation parameters were 5 V at a frequency of 12 Hz, a pulse width of 1 ms, and a duration of 1 minute. During tumescence, the maximal intracavernous pressure (ICP) was recorded. The total ICP was determined by the area under the curve from the beginning of cavernous nerve stimulation to a point 20 s after stimulus termination. Systemic blood pressure was measured by using a noninvasive tail-cuff system (Visitech Systems, Apex, NC, USA). The ratios of maximal ICP and total ICP (area under the curve) to mean systolic blood pressure (MSBP) were calculated to adjust for variations in systemic blood pressure, as previously described [5].
Primary culture for MCECs was done as previously described [15]. Briefly, the corpus cavernosum tissue was harvested from 8-week-old C57BL/6J mice and transferred into sterile vials containing Hank's balanced salt solution (Gibco, Carlsbad, CA, USA) and was washed two times in phosphate-buffered saline (PBS). The corpus cavernosum tissue was cut into two or three pieces and the samples were then plated on Matrigel stock solution (Becton Dickinson, Mountain View, CA, USA)-coated 60-mm cell culture dishes. The Matrigel was polymerized with a 5-minute incubation period at 37℃, and 3 ml of complement medium 199 (GIBCO), supplemented with 20% fetal bovine serum, 1% penicillin/streptomycin, 0.5 mg/ml heparin, and 5 ng/ml vascular endothelial growth factor-A, was added to the cell culture dish, and the dishes were then incubated at 37℃ with 5% CO2. After the cells were confluent and spread on the whole bottom of the dish (about 2 to 3 weeks after the start of culture), only sprouting cells were used for subcultivation. The sprouting cells were seeded onto dishes coated with 0.2% gelatin. Cells at passages between 2 and 3 were used for experiments.
The primary cultured cells were characterized as previously described [15]. Briefly, cells were cultured on sterile cover glasses (Marienfeld Laboratory, Lauda-Königshofen, Germany) that were placed on the bottom of 24-well plates. Individual chambers were incubated with antibody to platelet/endothelial cell adhesion molecule 1 (1:50; PECAM-1, an endothelial cell marker; Chemicon, Temecula, CA, USA), CD90 (1:50; a fibroblast marker; R&D Systems Inc., Minneapolis, MN, USA), or myosin (1:100; a smooth muscle cell marker; Sigma-Aldrich, St. Louis, MO, USA) overnight at 4℃ in a moist chamber. Mounting medium containing 4,6-diamidino-2-phenylindole (DAPI, Vector Laboratories Inc., Burlingame, CA, USA) was applied to the chamber and nuclei were labeled.
To examine the effect of the high-glucose condition with or without insulin treatment on Ninjurin 1 expression, the primary cultured MCECs were serum-starved for 24 hours and were then exposed to following conditions for 48 hours: normal glucose condition (5 mmol), high-glucose condition (30 mmol), or high-glucose condition (30 mmol)+insulin treatment (1 nM). Primary cultured MCECs incubated under each condition were used for Western blot analysis.
Equal amounts of protein (50 µg per lane) were electrophoresed on sodium dodecylsulfate-polyacrylamide gels (12%), transferred to nitrocellulose membranes, and probed with antibodies to Ninjurin 1 (BD Biosciences; Franklin Lakes, NJ, USA) or β-actin (1:6,000; Abcam, Cambridge, UK). The results were quantified by densitometry (n=4 per group).
For fluorescence microscopy (n=6 per group), the penis tissue was fixed in 4% paraformaldehyde for 24 hours at 4℃, and frozen tissue sections (8 µm thick) were incubated with antibodies to PECAM-1 (1:50; Chemicon), neurofilament (1:100; an axonal maker, Sigma-Aldrich), or Ninjurin 1 (1:50; Santa Cruz Biotechnology, Santa Cruz, CA, USA) at 4℃ overnight. After several washes with PBS, the sections were incubated with tetramethyl rhodamine isothiocyanate- or fluorescein isothiocyanate-conjugated secondary antibodies (Zymed Laboratories, South San Francisco, CA, USA) for 2 hours at room temperature. Signals were visualized, and digital images were obtained with a confocal microscope (FV1000, Olympus Co., Tokyo, Japan) under identical exposure settings.
Results are expressed as means±standard error. The group comparisons were made by Mann-Whitney rank-sum tests or Kruskal-Wallis tests. We performed statistical analysis with SigmaStat 3.5 (Systat Software Inc., Richmond, CA, USA). p-values less than 5% were considered significant.
Metabolic and physiologic variables, including body weight, blood glucose concentrations, and MSBP, are summarized in Table 1. Eight weeks after diabetes was induced by intraperitoneal injections of STZ, the fasting and postprandial blood glucose concentrations of the diabetic mice were significantly higher than those of the control mice. Body weight was significantly lower in the diabetic mice than in the controls (Table 1). Erectile function parameters, such as the ratios of maximal ICP and total ICP to MSBP, which were recorded at 5 V, were significantly lower in the diabetic mice than in the controls (Fig. 1). No significant difference in systemic blood pressure was noted between the groups (Table 1).
We performed fluorescent immunohistochemistry and Western blotting to evaluate the expression of Ninjurin 1 in the penile tissue of diabetic mice. Western blot analysis revealed a higher Ninjurin 1 protein expression in the penile tissue of diabetic mice than in that of controls (Fig. 2). Immunohistochemical staining also showed an increase in Ninjurin 1 expression in both corpus cavernosum tissue and the dorsal nerve bundle of diabetic mice compared with that in controls, whereas cavernous endothelial content (PECAM-1) and axonal content (neurofilament) in the dorsal nerve bundle were decreased by diabetes (Figs. 3, 4). Immunofluorescent double-staining revealed that the majority of Ninjurin 1 protein expression was overlapping with cavernous endothelial cells and with the dorsal nerve bundle (Figs. 3, 4).
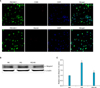
When we characterized the primary cultured cells, the majority of cells (more than 95%) showed positive staining for the endothelial cell marker (PECAM-1), but did not show positive staining for the smooth muscle cell marker myosin or the fibroblast marker CD90 (Fig. 5A). We determined the effect of the high-glucose condition on Ninjurin 1 expression in MCECs by Western blot. Ninjurin 1 expression was significantly increased by the high-glucose condition, and the levels were returned to baseline levels in MCECs exposed to the high-glucose condition and treated with insulin (Figs. 5B, 5C).
We report here that the expression of Ninjurin 1 protein was up-regulated in both cavernous endothelial cells and the dorsal nerve bundle of the diabetic mice. In the in vitro study in MCECs, Ninjurin 1 expression was also increased by the high-glucose condition and was returned to baseline levels by treatment with insulin.
Peripheral neuropathy of the autonomic nerve and endothelial dysfunction are important causes of diabetic ED. The reduction in neuronal nitric oxide synthase-containing nerve fiber and endothelial NOS activity, impaired penile angiogenesis, and the subsequent impairment in neurogenic- and endothelium-mediated smooth muscle relaxation are major contributing factors to diabetic ED [5,6,16,17]. Therefore, therapies aimed at restoring both nerve and endothelial function may be a promising therapeutic strategy in patients with ED associated with diabetes.
In the present study, Western blot analysis revealed higher Ninjurin 1 expression in the penile tissue of diabetic mice than in that of age-matched controls. Moreover, immunohistochemical staining showed higher Ninjurin 1 expression in the dorsal nerve bundle of diabetic mice than in that of controls, whereas axonal content, as determined by neurofilament immunohistochemistry, was decreased by diabetes. Previous study also reported an increase in Ninjurin 1 expression after peripheral nerve injury, with the peak level of expression occurring 7 to 14 days after injury [12]. Several lines of evidence suggest that blood-borne immune cells play an important role in the onset and progression of CNS-related disease, such as multiple sclerosis, an autoimmune inflammatory disease in the CNS [18,19]. A recent study reported in an experimental autoimmune encephalomyelitis model, an animal model for multiple sclerosis, that Ninjurin 1 was highly expressed in myeloid cells (macrophage/monocyte and neutrophils), which enhances penetration of these cells into the blood-brain barrier and induces neuroinflammation and nerve damage [13]. The authors from this study suggested that Ninjurin 1 could be a novel therapeutic target for neuroinflammatory disease related with immune cells. Besides diabetic neuropathy, cavernous nerve injury is a major cause of radical prostatectomy-induced ED [20,21]. Cavernous nerve injury induces local inflammation and immune responses and finally contributes to the development of ED [22-24]. Therefore, it is necessary to examine the effects of the blockade of Ninjurin 1 on erectile function in animal models for diabetes or cavernous nerve injury.
Ninjurin 1 is also known to play a crucial role in vascular homeostasis during the embryonic period [14]. In the present study, the expression of Ninjurin 1 protein was significantly increased in both cavernous endothelial cells in vivo and primary cultured MCECs exposed to a high-glucose condition in vitro. Ang1 is the ligand of the Tie2 receptor tyrosine kinase and plays an important role in angiogenesis and maturation of blood vessels [25]. Ang1 is also known as a survival factor for vascular endothelial cells [25]. In contrast, Ang2 is an antagonist for Ang1 on the Tie2 receptor and is known to induce endothelial apoptosis through inhibition of survival signal, such as the phosphatidylinositol 3-kinase/Akt pathway [25]. A recent study reported that Ninjurin 1 is a critical mediator for the regression of the hyaloids vascular system during the developmental period by regulation and Ang1/Ang2 balance, i.e., decrease in Ang1 expression and increase in Ang2 expression, whereas Ninjurin 1 neutralizing antibody inhibited regression of the hyaloids vascular system [14].
Collectively, these findings suggest that Ninjurin 1 is involved in both neuropathy and angiopathy, which are important for the pathogenesis of diabetic ED. Therefore, further studies are needed to test whether inhibition of the Ninjurin 1 pathway can restore erectile function by reestablishing penile neural and microvascular structures.
We for the first time have reported an up-regulation of Ninjurin 1 protein in the penis of diabetic mice. The expression of Ninjurin 1 protein was significantly induced in both cavernous endothelial cells and the dorsal nerve bundle of the diabetic mice in vivo and in MCECs exposed to the high-glucose condition in vitro. With regard to the role of Ninjurin 1 in neuropathy and vascular regression, it would be interesting to examine the effects of inhibition of Ninjurin 1 on erectile function in animal models of ED of vascular or neurogenic causes.
Figures and Tables
FIG. 1
Decrease in erectile function in diabetic mice. (A) Representative intracavernous pressure (ICP) responses to electrical stimulation of the cavernous nerve for the age-matched control and diabetic groups. The stimulus interval is indicated by a solid bar. (B) The ratios of mean maximal ICP and total ICP to mean systolic blood pressure (MSBP) stimulated at 5 V and 12 Hz in each group. Each bar depicts the mean values (±standard error) from n=6 animals per group. DM, diabetes mellitus. a:p<0.01 compared with the control group.

FIG. 2
Increase in Ninjurin 1 expression in diabetic mouse penis. (A) Representative Western blot analysis showing the protein expression of Ninjurin 1. (B) Data are presented as the relative density of Ninjurin 1 protein compared with that of β-actin. The relative ratio measured in the control group is arbitrarily presented as 1. Each bar depicts the mean values (±standard error) from n=4 animals per group. DM, diabetes mellitus. a:p<0.01 compared with the control group.
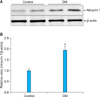
FIG. 3
Differential distribution of Ninjurin 1 in the corpus cavernosum tissue of diabetic mice. Immunofluorescent double-staining of penile tissue performed with antibody to PECAM-1 (an endothelial cell marker) and Ninjurin 1. Note increase in Ninjurin 1 expression and decrease in PECAM-1 expression in diabetic corpus cavernosum tissue (×200). DM, diabetes mellitus; PECAM-1, platelet/endothelial cell adhesion molecule-1.
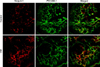
FIG. 4
Differential distribution of Ninjurin 1 in the dorsal nerve bundle of diabetic mice. Immunofluorescent double-staining of penile tissue performed with antibody to neurofilament (an axonal marker) and Ninjurin 1. Note increase in Ninjurin 1 expression and decrease in nerve content in diabetic dorsal nerve bundle (×400). DM, diabetes mellitus; DAPI, 4,6-diamidino-2-phenylindole.
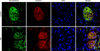
FIG. 5
Increase in Ninjurin 1 expression in mouse cavernous endothelial cells exposed to the high-glucose (HG) condition, which was reversed by treatment with insulin. (A) Characterization of primary cultured mouse cavernous endothelial cells. Fluorescent immunocytochemistry of primary mouse cavernous endothelial cells with antibody against platelet/endothelial cell adhesion molecule-1 (PECAM-1) (positive marker) and antibody against CD90 and myosin (negative markers) (×200). (B) Representative Western blot analysis showing the protein expression of Ninjurin 1. After serum starvation for 24 hours, cavernous endothelial cells were incubated under the following conditions for 48 hours: normal glucose condition (NG, 5 mM); high-glucose condition (30 mM); high-glucose condition (30 mM)+insulin (IN, 1 nM). (C) Data are presented as the relative density of Ninjurin 1 protein compared with that of β-actin. The relative ratio measured in the normal glucose condition is arbitrarily presented as 1. Each bar depicts the mean values (±standard error) from four independent experiments. DAPI, 4,6-diamidino-2-phenylindole. a:p<0.01 compared with the other groups, b:p<0.01 compared with HG group.
ACKNOWLEDGEMENTS
This study was supported by a grant of the Korea Healthcare technology R&D Project, Ministry for Health, Welfare & Family Affairs (Jun Kyu Suh, A110076), Republic of Korea.
References
1. Malavige LS, Levy JC. Erectile dysfunction in diabetes mellitus. J Sex Med. 2009. 6:1232–1247.
2. Hakim LS, Goldstein I. Diabetic sexual dysfunction. Endocrinol Metab Clin North Am. 1996. 25:379–400.
3. Burchardt T, Burchardt M, Karden J, Buttyan R, Shabsigh A, de la Taille A, et al. Reduction of endothelial and smooth muscle density in the corpora cavernosa of the streptozotocin induced diabetic rat. J Urol. 2000. 164:1807–1811.
4. Ahn GJ, Sohn YS, Kang KK, Ahn BO, Kwon JW, Kang SK, et al. The effect of PDE5 inhibition on the erectile function in streptozotocin-induced diabetic rats. Int J Impot Res. 2005. 17:134–141.
5. Jin HR, Kim WJ, Song JS, Choi MJ, Piao S, Shin SH, et al. Functional and morphologic characterizations of the diabetic mouse corpus cavernosum: comparison of a multiple low-dose and a single high-dose streptozotocin protocols. J Sex Med. 2009. 6:3289–3304.
6. El-Sakka AI, Lin CS, Chui RM, Dahiya R, Lue TF. Effects of diabetes on nitric oxide synthase and growth factor genes and protein expression in an animal model. Int J Impot Res. 1999. 11:123–132.
7. Zhang XH, Filippi S, Morelli A, Vignozzi L, Luconi M, Donati S, et al. Testosterone restores diabetes-induced erectile dysfunction and sildenafil responsiveness in two distinct animal models of chemical diabetes. J Sex Med. 2006. 3:253–264.
8. Rendell MS, Rajfer J, Wicker PA, Smith MD. Sildenafil Diabetes Study Group. Sildenafil for treatment of erectile dysfunction in men with diabetes: a randomized controlled trial. JAMA. 1999. 281:421–426.
9. Martínez-Jabaloyas JM, Gil-Salom M, Villamon-Fort R, Pastor-Hernandez F, Martinez-Garcia R, Garcia-Sisamon F. Prognostic factors for response to sildenafil in patients with erectile dysfunction. Eur Urol. 2001. 40:641–646.
10. Musicki B, Burnett AL. Endothelial dysfunction in diabetic erectile dysfunction. Int J Impot Res. 2007. 19:129–138.
11. De Young L, Yu D, Bateman RM, Brock GB. Oxidative stress and antioxidant therapy: their impact in diabetes-associated erectile dysfunction. J Androl. 2004. 25:830–836.
12. Araki T, Milbrandt J. Ninjurin, a novel adhesion molecule, is induced by nerve injury and promotes axonal growth. Neuron. 1996. 17:353–361.
13. Ifergan I, Kebir H, Terouz S, Alvarez JI, Lecuyer MA, Gendron S, et al. Role of Ninjurin-1 in the migration of myeloid cells to central nervous system inflammatory lesions. Ann Neurol. 2011. 70:751–763.
14. Lee HJ, Ahn BJ, Shin MW, Jeong JW, Kim JH, Kim KW. Ninjurin1 mediates macrophage-induced programmed cell death during early ocular development. Cell Death Differ. 2009. 16:1395–1407.
15. Yin GN, Ryu JK, Kwon MH, Shin SH, Jin HR, Song KM, et al. Matrigel-based sprouting endothelial cell culture system from mouse corpus cavernosum is potentially useful for the study of endothelial and erectile dysfunction related to high-glucose exposure. J Sex Med. 2012. 9:1760–1772.
16. Saenz de Tejada I, Goldstein I, Azadzoi K, Krane RJ, Cohen RA. Impaired neurogenic and endothelium-mediated relaxation of penile smooth muscle from diabetic men with impotence. N Engl J Med. 1989. 320:1025–1030.
17. Cellek S, Rodrigo J, Lobos E, Fernández P, Serrano J, Moncada S. Selective nitrergic neurodegeneration in diabetes mellitus : a nitric oxide-dependent phenomenon. Br J Pharmacol. 1999. 128:1804–1812.
18. McFarland HF, Martin R. Multiple sclerosis: a complicated picture of autoimmunity. Nat Immunol. 2007. 8:913–919.
19. Brochard V, Combadiere B, Prigent A, Laouar Y, Perrin A, Beray-Berthat V, et al. Infiltration of CD4+ lymphocytes into the brain contributes to neurodegeneration in a mouse model of Parkinson disease. J Clin Invest. 2009. 119:182–192.
20. Walsh PC, Mostwin JL. Radical prostatectomy and cystoprostatectomy with preservation of potency. Results using a new nerve-sparing technique. Br J Urol. 1984. 56:694–697.
21. Noldus J, Michl U, Graefen M, Haese A, Hammerer P, Huland H. Patient-reported sexual function after nerve-sparing radical retropubic prostatectomy. Eur Urol. 2002. 42:118–124.
22. Catalona WJ, Bigg SW. Nerve-sparing radical prostatectomy: evaluation of results after 250 patients. J Urol. 1990. 143:538–543.
23. Catalona WJ, Basler JW. Return of erections and urinary continence following nerve sparing radical retropubic prostatectomy. J Urol. 1993. 150:905–907.
24. Kim SW. Prostatic disease and sexual dysfunction. Korean J Urol. 2011. 52:373–378.
25. Augustin HG, Koh GY, Thurston G, Alitalo K. Control of vascular morphogenesis and homeostasis through the angiopoietin-Tie system. Nat Rev Mol Cell Biol. 2009. 10:165–177.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download