Abstract
Incidence of nontuberculous mycobacterium (NTM) pulmonary disease is increasing with the wider recognition and development of diagnostic technology. Mycobacterium kansasii is the second most common pathogen of NTM pulmonary disease in human immunodeficiency virus (HIV)-infected patients. However in Korea, the incidence of M. kansasii pulmonary disease is relatively low, and there has been no report of M. kansasii pulmonary disease with bronchial involvement in HIV patients, to the best of our knowledge. We report a case of M. kansasii pulmonary disease presenting with endobronchial lesions in an HIV-infected patient complaining of chronic cough with bilateral enlargements of hilar lymph nodes on chest X-ray.
Although nontuberculous mycobacterium (NTM) infection is the major opportunistic infection in immunodeficient patients such as human immunodeficiency virus (HIV)-infected patients, recently it has been increasing in immunocompetent HIVpatients. Major pathogens of NTM pulmonary disease are different according to region and time. Mycobacterium avium complex is the most common pathogen, followed by Mycobacterium kansasii, and Mycobacterium abscessus in the United States and Japan1, but in Korea, followed by M. abscessus and Mycobacterium fortuitum complex2.
Unlike other NTMs, M. kansasii is found only in the city tap water systems, not in the natural environment such as soil or rivers and M. kansasii pulmonary disease occurs mainly in urban residents3. In Korea, there have been few cases of M. kansasii pulmonary disease and no report of M. kansasii pulmonary disease with bronchial involvement.
In this report, we describe a case of M. kansasii pulmonary disease presenting with endobronchial lesions in a HIV-infected patient and review relevant literature.
A 39-year-old male presented with cough and blurred vision for 1 month. Six years ago he was diagnosed with HIV infection in another hospital and he was followed regularly at public health care facility. He had no previous history of pulmonary tuberculosis or other pulmonary diseases. He was a nonsmoker and nondrinker. Forty days ago before admission, he visited the other hospital due to blurred vision and cough for 1 month. CD4 cell counts were 3/mm3 and highly active antiretroviral therapy with combivir and kaletra was started with the concurrent treatment for cytomegalovirus retinitis. He was transferred to our hospital for further evaluation of chronic cough with an abnormal chest X-ray.
The patient had a chronically ill appearing and vital signs were stable. There were no specific findings in head and neck area. Breathing sounds were clear and there were no signs of abdominal tenderness. A complete blood count showed white blood cell count of 9,390/mm3 (neutrophil 61.0%, lymphocyte 21.0%, eosinophil 7.0%), hemoglobin 11.3 g/dL, and platelet 322×109/L with C-reactive protein of 3.62 mg/dL (normal, <0.5 mg/dL). A blood chemistry analysis was unremarkable. CD4 count was 247/mm3, and HIV viral load was 2,499 copies/mL. Tuberculin skin test revealed a negative result with a positive interferon-gamma release assay result. Initial chest X-ray showed bilateral hilar prominence, raising the possibility of bilateral hilar lymphadenopathy (Figure 1A). Chest computed tomography revealed multifocal nodular consolidations with some surrounding ground glass opacities in both lungs. There were several enlarged lymph nodes with some internal low-attenuated lesions in the paratracheal, subcarinal, and both hilar regions (Figure 2). Bronchoscopy showed an elevated nodular lesion with central whitish necrosis at apical segment of right upper lobe and luminal narrowing at apical part of apicoposterior segment of left upper lobe (Figure 3). Bronchial mucosal biopsy was performed at the both above segments. As bronchial washing fluids smear was positive acid fast bacilli, we started with standard antituberculous medication empirically. However, NTM was detected by polymerase chain reaction from the bronchial specimens and histologic findings at bronchial mucosa revealed chronic granulomatous inflammations with positive Ziehl-Neelsen stain (Figure 4). One month later, M. kansasii was isolated in bronchial washing fluids and bronchial mucosa specimens. Finally, we diagnosed M. kansasii pulmonary disease with bronchial involvement and he was treated with isoniazid at 300 mg, rifabutin at 150 mg, and ethambutol at 1,200 mg. After the medication, his cough improved gradually and a chest X-ray after 18 months of treatment showed complete regression of the bilateral hilar lymphadenopathy (Figure 1B).
M. kansasii infection and its associated pulmonary disease mainly develop in urban residents of industrialized cities3. In Korea, as the incidence of M. kansasii pulmonary disease is rare, there has been one case report in immunodeficient patients and three cases in immunocompetent patients, to our knowledge4,5.
M. kansasii primarily affects middle-aged men, and the risk factors for it include pneumoconiosis, chronic obstructive lung disease, previous mycobacterial disease, malignancy, and immunocompromised condition6. In the current case, it is assumed that the patient developed NTM pulmonary disease as an opportunistic infection due to an immunocompromised condition, i.e., HIV infection.
According to 2007 American Thoracic Society guidelines, the diagnosis of nontuberculous mycobacterium disease requires clinical and radiologic signs of pulmonary disease, and bacterial evidence (≥two times positive sputum cultures or one time positive bronchial washing fluid culture), and pathologic evidence of mycobacterial activity1. The patient in the current case was admitted for respiratory symptom of cough for one month with abnormal radiologic findings. M. kansasii was cultured in bronchial washing specimen and mucosal tissue and pathologic findings showed chronic granulomatous inflammations with positive Ziehl-Neelsen stain. As these findings fulfilled the diagnostic requirements for nontuberculous mycobacterial disease, therefore we diagnosed M. kansasii pulmonary disease presenting concurrently with endobronchial lesions.
Endobronchial involvement due to M. kansasii is rare and only few cases have been reported in the worldwide7,8, and the prevalence is unknown. Moreover, most of the patients reported were infected with HIV and only one was an immunocompetent host. However, there has been no report of M. kansasii pulmonary disease with bronchial involvement in Korea. Although the mechanism of bronchial involvement in NTM pulmonary disease is still unknown, it could be similar to endobronchial tuberculosis9; 1) via recurrent dissemination of tuberculosis from the peripheral cavitary lesion towards central bronchus, 2) through direct bronchial invasion by adjacent mediastinal lymph node already infected, 3) transmission in a hematogenous spread, 4) transmission of tuberculosis from upper to peripheral airway as in a case with laryngeal tuberculosis, 5) co-infection of both lung parenchyme and bronchus in the very first place. In the present case, we assume that it occurred by direct bronchial invasion from an adjacent mediastinal lymph node, considering the radiologic findings.
According to the 2007 American Thoracic Society recommendation, treatment for M. kansasii pulmonary disease in adults consist of isoniazid (300 mg), rifampin (600 mg), and ethambutol (25 mg/kg for the first 2 months, then 15 mg/kg) given daily for 18 months with at least 12 months of negative sputum cultures. We used rifabutin instead of rifampin in this case because of drug interaction with kaletra, a type of protease inhibitor. Treatment regimens for M. kansasii pulmonary disease with HIV patients should follow the guidelines for treatment of non-HIV-infected persons, with the consideration for a longer duration of treatment1.
In summary, we report an uncommon case of HIV-infected patient with M. kansasii pulmonary disease simultaneously developed as endobronchial involvement and improved after treatment. Clinician should keep in mind that M. kansasii pulmonary disease can present with endobronchial lesion, especially in an immunocompromised host.
Figures and Tables
Figure 1
Initial chest X-ray shows bilateral hilar prominence, raising the possibility of bilateral hilar lymphadenopathy. (B) Follow-up chest X-ray after 18 months-therapy of Mycobacterium kansasii infection shows a complete regression of bilateral hilar lymphadenopathy.
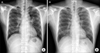
Figure 2
Chest computed tomography scan shows multifocal nodular consolidations with some surrounding ground glass opacities in both lungs and several enlarged lymph nodes with some internal low-attenuated lesions in both hilar regions.

References
1. Griffith DE, Aksamit T, Brown-Elliott BA, Catanzaro A, Daley C, Gordin F, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007; 175:367–416.
2. Koh WJ, Kwon OJ, Jeon K, Kim TS, Lee KS, Park YK, et al. Clinical significance of nontuberculous mycobacteria isolated from respiratory specimens in Korea. Chest. 2006; 129:341–348.
3. Griffith DE. Management of disease due to Mycobacterium kansasii. Clin Chest Med. 2002; 23:613–621.
4. Park SY, Park JH, Jegal YJ, Lee JH, Lim CM, Lee SD, et al. A case of idiopathic CD4+ T-lymphocytopenia with disseminated Mycobacterium kansasii infection and pulmonary alveolar proteinosis. Tuberc Respir Dis. 2000; 48:377–382.
5. Koh WJ, Kwon OJ, Suh GY, Chung MP, Kim H, Lee NY, et al. A case report of three patients with nontuberculous mycobacterial pulmonary disease caused by Mycobacterium kansasii. Tuberc Respir Dis. 2003; 54:459–466.
6. Maliwan N, Zvetina JR. Clinical features and follow up of 302 patients with Mycobacterium kansasii pulmonary infection: a 50 year experience. Postgrad Med J. 2005; 81:530–533.
7. Connolly MG Jr, Baughman RP, Dohn MN. Mycobacterium kansasii presenting as an endobronchial lesion. Am Rev Respir Dis. 1993; 148:1405–1407.
8. Manali ED, Tomford WJ, Liao DW, Farver C, Mehta AC. Mycobacterium kansasii endobronchial ulcer in a nonimmunocompromised patient. Respiration. 2005; 72:305–308.
9. An JY, Lee JE, Park HW, Lee JH, Yang SA, Jung SS, et al. Clinical and bronchoscopic features in endobronchial tuberculosis. Tuberc Respir Dis. 2006; 60:532–539.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download