Abstract
Giant multilocular prostatic cystadenoma (GMPC) is a rare benign tumor involving the prostate gland. Microscopically, it masquerades phyllodes tumor or transitional zone hyperplasia. We report one case of GMPC arising from the prostate central zone (CZ), presenting with long-standing aspermia associated with seminal vesicle fibrous obliteration.
Giant multilocular prostatic cystadenoma (GMPC), a rare benign tumor surrounding the prostate gland, is seldom reported to occur within the gland. GMPC is usually characterized by large, multilocular cysts that originate from the prostate itself,1,2 as prostate pedicles,3 or completely separated from the gland and located between the rectum and bladder.4-8 In general, diagnosis of GMPC is not complicated, but requires histologic evaluation to differentiate it from phyllodes tumor and nodular hyperplasia, which predominantly occur in the transitional zone (TZ). We report a case of GMPC arising from the central zone (CZ) and presenting with long-standing aspermia. The vas deferens and seminal vesicles showed near complete fibrous obliteration with a relatively thick external muscle proper. The verumontanum outflow obstruction, which caused aspermia, has not previously been described as a manifestation of GMPC.
A 61-year-old male presented with a 1-year history of abdominal distension and urinary retention, and a 3-year history of aspermia. On digital rectal examination, the distended bladder was palpable in the anterior pelvis, along with an enlarged prostate. Bladder scan showed residual urine greater than 999mL, which was later found to be cystic fluids associated with GMPC. Pelvic computed tomography and magnetic resonance imaging revealed a 14 × 14-cm mass located between the rectum and bladder (Fig. 1A). Serum prostate-specific antigen (PSA) level was elevated (38.2ng/mL). Mass excision was performed for pelvic cyst of unknown origin.
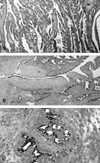
Upon surgical exploration, a sizeable globular mass directly attached to the prostate base was found. The mass displaced the bladder anteriorly and both seminal vesicles were unrecognizable. No connection to the proximal urethra was identified; the rectum and bladder did not appear to be involved. Gross examination revealed a 9 × 7-cm, firm, rubbery, tannish-gray mass with clearly delineated yet tightly adjoined solid and cystic components attached to fibrous tissue (Fig. 1B). An adjoining multilocular cystic mass ruptured during surgery. Microscopically, the mass appeared to originate from the CZ, in close proximity to the vas deferens and seminal vesicles. Due to distension and stretching, parts of the large cyst remained as flat mono-layered epithelial cells and had paper-thin fibrous walls. The tandem arrayed prominent intraluminal ridges and extensive arborizing complex glands were noteworthy (Fig. 2A). The hallmark lobular pattern or eccentrically curvilinear branched hyperplastic glands, usually seen in conventional nodular hyperplasia, were not identified. Urothelium, representing either TZ or prostate urethra, was not found. Focal areas of the solid mass had leaflets with profound stromal cells projecting into the cystic cavity, which mimicked phyllodes tumor (Fig. 2B). Intraluminal snouting and tufts were occasionally seen, however, cellular atypia typically found in prostatic intraepithelial neoplasia (PIN) was not present. There was no cytological atypia or appreciable mitosis. Epithelial cells were strongly immunoactive to PSA. Seminal vesicles showed marked luminal obliteration by fibromuscular hypertrophy and periluminal amyloidosis (Fig. 2C), which was not previously noticeable on radiological images and gross examination.
One year after surgery, the patient is alive with no evidence of disease.
Microscopically, GMPC differs from conventional TZ hyperplasia in several aspects. First, GMPC involves the CZ. Second, glandular branching directions are different; glandular branching in GMPC is compact, and hyperplastic glands radiate in all directions from the central lumen as subsidiary ducts. TZ hyperplasia forms well-circumscribed expansive nodules produced by the budding and branching of newly formed ductal-acinar structures, with an eccentric branched curvilinear appearance generally less than 8mm.9 Nodular hyperplasia can mimic phyllodes tumor, which forms leaflet-like projections with increased stromal cellularity. The major difference between phyllodes tumor and hyperplasia is the stromal cellularity, however these entities lie on a continuous spectrum. Reviews of phyllodes tumors report that nearly all cases arise centrally near the verumontanum or in the lateral portion of the TZ.10 In spite of prominent stromal participation with cellular atypia, phyllodes tumor rarely leads to recurrence or metastasis.9
In our case, the patient's main symptom was aspermia, likely caused by a compressed seminal vesicle. The seminal vesicle is composed of 2 distinct muscle propers: a thin external longitudinal and a thicker internal circular muscle layer.9 In our case, portions of the vas deferens and seminal vesicles showed nearly complete fibrous obliteration and a relatively thick external muscle proper.
This pelvic mass apparently originated from the prostate, due to the intense immunoreactivity to PSA. The elevated PSA in the present case suggests microvasculature influx, such as rupture from the markedly dilated cystic glands. In a few documented cases,1,2,4,5 PSA level was not proportionally related (CC = 0.25) to mass volume (Table 1). Therefore, further studies regarding the interrelationship between tumor volume and PSA level are required.
In conclusion, we report a case of GMPC involving the CZ and presenting with aspermia, suggesting involvement of the compressed seminal vesicles.
Figures and Tables
Fig. 1
Preoperative MRI of the mass on vertical view (A) and gross section of the cystic and solid mass (B). Urinary bladder (*) was compressed and moved anteriorly (dotted arrow: solid mass, double-headed arrow: junction of solid and cystic mass, solid arrow: cystic mass).
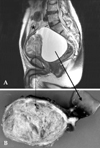
Fig. 2
Microscopic finding of giant multilocular cystadenoma. (A) Tandem arrayed prominent intraluminal ridges and extensive arborizing complex glands. (B) Increased stromal cellularity projecting into dilated cystic space, giving the impression of mammary gland phyllodes tumor. (C) Seminal vesicle was obliterated by periluminal amyloidosis.
References
1. Kirsch AJ, Newhouse J, Hibshoosh H, O'Toole K, Ritter J, Benson MC. Giant multilocular cystadenoma of the prostate. Urology. 1996. 48:303–305.

2. Seong BM, Cheon J, Lee JG, Kim JJ, Chae YS. A case of multilocular prostatic cystadenoma. J Korean Med Sci. 1998. 13:554–558.

3. Levy DA, Gogate PA, Hampel N. Giant multilocular prostatic cystadenoma: a rare clinical entity and review of the literature. J Urol. 1993. 150:1920–1922.

4. Maluf HM, King ME, DeLuca FR, Navarro J, Talerman A, Young RH. Giant multilocular prostatic cystadenoma: a distinctive lesion of the retroperitoneum in men. A report of two cases. Am J Surg Pathol. 1991. 15:131–135.
5. Matsumoto K, Egawa S, Iwabuchi K, Baba S. Prostatic cystadenoma presenting as a large multilocular mass. Int J Urol. 2002. 9:410–412.

6. Allen EA, Brinker DA, Coppola D, Diaz JI, Epstein JI. Multilocular prostatic cystadenoma with high-grade prostatic intraepithelial neoplasia. Urology. 2003. 61:644.

7. Choi YH, Namkung S, Ryu BY, Choi KC, Park YE. Giant multilocular prostatic cystadenoma. J Urol. 2000. 163:246–247.

8. Rusch D, Moinzadeh A, Hamawy K, Larsen C. Giant multilocular cystadenoma of the prostate. AJR Am J Roentgenol. 2002. 179:1477–1479.

9. McNeal JE. Stenberg SS, editor. Prostate. Histology for pathologists. 1997. 2nd ed. Philadelphia, PA: Lippincott-Raven;997–1017.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download