Abstract
Objective
To evaluate the efficacy and safety of radiofrequency ablation (RFA) for low-risk papillary thyroid microcarcinoma (PTMC) in a large population.
Materials and Methods
Cases of 152 biopsy-proven PTMCs from 133 patients who had undergone RFA for PTMC between May 2008 and January 2017 were included in this study. All patients were either of high surgical risk or refused to undergo surgery. They were followed up for at least 6 months after initial RFA. Ultrasonography (US) and computed tomography were performed to evaluate the PTMC and the presence of neck metastasis before treatment. RFA was conducted using an internally cooled thyroid-dedicated electrode system. Follow-up US was performed at 1 week, and 2, 6, and 12 months, after the initial RFA, and then at every 6–12 months. We evaluated serial changes of ablated tumors, newly developed cancers, lymph node (LN) or distant metastasis and complications.
Results
Complete disappearance was found in 91.4% (139/152) of ablated tumors. Among the 13 tumors in patients who did not show complete disappearance, no tumor displayed any regrowth of the residual ablated lesion during the follow-up period. The mean follow-up period was 39 months. During the follow-up period, there were no local recurrence, no LN or distant metastasis, and no newly developed thyroid cancers. No patients were referred to surgery. The overall complication rate was 3% (4/133) of patients, including one voice change. There were no life-threatening complications or procedure-related deaths.
Although papillary thyroid microcarcinoma (PTMC) is the most indolent type of thyroid cancer, with a good prognosis and low mortality rate, surgery has been the mainstream treatment (12). However, the 2015 American Thyroid Association (ATA) guidelines suggest that active surveillance could be the first-line management used for low-risk PTMC (1). Although studies on these ablation techniques show favorable outcomes with low complication rates, they have several drawbacks such as small patient numbers and short follow-up periods (3).
Recently, radiofrequency ablation (RFA), laser ablation (LA), and microwave ablation (MWA) have been used as first-line treatments for primary low-risk PTMCs without evidence of gross extrathyroidal extension, lymph node (LN) metastasis, or metastasis beyond the neck (456789). Although studies on these ablation techniques show favorable outcomes with low complication rates, they have drawbacks in that they contain small patient numbers and have short follow-up periods. For example, the study with the largest population of 92 patients, who were treated with RFA reported excellent local tumor ablation, but the follow-up period was too short, 7.8 months (5). Another multicenter study with longer follow-up, 4 years, also showed excellent local control, but this study enrolled only six patients (6). Therefore, the purpose of our study was to evaluate the efficacy and safety of RFA for low-risk PTMC in a large patient population with a longer follow-up period.
This retrospective study was approved by our Institutional Review Board for human investigations, and written informed consent was obtained from all patients before the RFA was conducted.
Between May 2008 and January 2017, 155 patients with primary PTMCs were treated with ultrasonography (US)-guided RFA at two institutions. Patients' inclusion criteria were: 1) they had PTMCs (0.3 ≤ size < 1 cm) confirmed by US-guided biopsy, of 0.3 cm size (1011); 2) no evidence of gross extrathyroidal extension or metastasis on both US and contrast-enhanced neck computed tomography (CT) (121314); 3) either multiple or solitary PTMCs; and 4) they had medical contraindications for surgery (e.g., old age: > 80 years or a co-morbidity such as cardiovascular disease, history of stroke, central nervous system vascular malformation, other malignancy, and immunocompromised state) or refused surgery. Since we routinely performed the hydro dissection technique to ensure safety during RFA, the tumors in the danger triangles could also be ablated if these inclusion criteria were fulfilled.
Patients were excluded for any of the following criteria: 1) thyroid cancer with gross extrathyroidal extension; 2) LN metastasis; 3) metastasis beyond the neck; and 4) pregnancy. In addition, six PTMCs in two patients were excluded due to follow-up loss after RFA. Finally, 133 patients were enrolled in this study (Fig. 1).
All patients were evaluated by US examination using either an iU22 US (Philips Healthcare, Bothell, WA, USA) or EUB-7500 (Hitachi Medical Systems, Tokyo, Japan) US unit, each of which was equipped with a linear high-frequency probe (5–14 MHz). US examination was followed by US-guided biopsy for histopathological confirmation. The diameters (the largest diameter and two other perpendicular diameters) and tumor volume of each nodule were evaluated on US examination. The volume of each tumor was calculated as V = πabc/6 (where V is the volume, a is the largest diameter, and b and c are the two other perpendicular diameters) (15).
CT was performed in all patients to exclude metastasis. Laboratory examinations, including measurements of thyroid function, serum thyroglobulin, thyroglobulin antibody, platelet count, and blood coagulation tests were performed.
All patients' medical records, their radiological information such as US and CT images, and the results of their laboratory tests were reviewed by one radiologist with 17 years of experience in thyroid imaging and thyroid biopsy.
All RFA procedures were performed by one expert radiologist with 11 years of experience in performing thyroid RFA on an outpatient basis. An SSP-2000 (Taewoong Medical, Gimpo, Korea) or VIVA RF System (STARmed, Goyang, Korea) radiofrequency generator and an 18-gauge thyroid-dedicated internally cooled electrode with 0.5, 0.7, and 1 cm active tips were used (Well-point RF electrode, STARmed; VIVA RF System), depending on the size of the targeted tumor (16). Patients were placed in the supine position with their neck extended, and two grounding pads were attached to both thighs.
To prevent serious hemorrhage, blood vessels along the approach route were carefully evaluated by Doppler US. The relationships of the tumor with anatomically critical structures such as nerves, esophagus, and trachea were carefully evaluated to prevent injury. Patients were injected with 1% lidocaine for local anesthesia at the puncture site and around the thyroid capsule (Fig. 2). Hydrodissection technique was used to prevent thermal injury and to obtain a safety margin, for which a 25-gauge needle was used to inject a cold dextrose solution between the tumor and the structure (17).
A trans-isthmic approach was used for the RFA (918). The initial radiofrequency (RF) power ranged from 15 to 40 W, according to the size of the electrode tip. If a transient hyperechoic zone did not form at the electrode tip within 5–10 seconds, the RF power was increased in 5 to 10 W increments, to 25–50 W. During the ablation of small tumors, the electrode tip was fixed in the center of the tumor and not moved during the ablation. However, relatively larger tumors were treated unit-by-unit, using a moving-shot technique (15192021). After complete ablation of the tumor itself, a sufficient quantity of adjacent normal thyroid tissue was also ablated, by at least 2 mm, to obtain a safety margin and prevent a marginal recurrence. Meanwhile, the presence of any complications was carefully evaluated during and immediately after ablation according to the clinical signs and symptoms.
Patients were assessed by US, CT, and clinical evaluation. Follow-up US was performed at 1 week, and 2, 6, and 12 months and then at every 6–12 months. On the follow-up US examinations, the tumor volume, and its largest diameter, and the development of new tumors and LN metastasis were monitored. We performed US-guided biopsy if an abnormal finding appeared in US and CT, suggesting regrowth of the residual ablated lesion, other new nodules separated from an initial ablated tumor, new LN metastasis, or distant metastasis. The percentage volume reduction was calculated as follows: volume reduction ratio (VRR) = ([initial volume - final volume] × 100) / initial volume (15). Contrast-enhanced neck CT was performed in all patients at 2 and 4 years after the ablation to evaluate LN metastasis. A thyroid function test was performed immediately after the ablation, and at 2, 6, and 12 months after the ablation.
Additional RFA was performed in cases with insufficient safety margins or new cancer detection on follow-up. The efficacy of treatment was evaluated using several parameters: change in volume, volume reduction, and complete disappearance (20). Complications during the procedure or follow-up were assessed using the reporting standards of the Society of Interventional Radiology (22). Early complications, within 30 days after the RFA, and delayed, complications 30 days after RFA, were evaluated (23).
Table 1 lists the characteristics of the enrolled patients, PTMCs, and RFA treatment parameters. A total of 133 patients, with 152 tumors were enrolled in this study (Fig. 1). Fifty-four tumors in 49 patients were followed up for more than 4 years. The mean largest tumor diameter was 0.43 ± 0.14 cm (range, 0.30–0.95 cm). Among the 152 tumors, 111 had the largest diameter less than 0.5 cm, while the remaining 41 tumors were 0.5 cm or larger (Table 1). The mean tumor volume was 0.03 ± 0.04 mL (0.001–0.320 mL).
The mean number of RFA sessions per tumor was 1.1 (range, 1–2); 137 tumors were treated in one RF session, while 15 tumors were treated in two RF sessions owing to insufficient safety margins after the first ablation. The mean time interval between the first and second RFA was 7 ± 2 months (range, 3–12 months). The mean follow-up duration after the RFA was 39 ± 25 months (range, 6–104 months).
The efficacy of RFA is detailed in Table 2; 139 of the total 152 tumors (91.4%), 104 of 111 tumors less than 0.5 cm (93.7%), and 35 of 41 tumors greater than 0.5 cm (85.4%) completely disappeared. Of these, 108 of 152 total tumors (71.1 %), 83 of 111 tumors less than 0.5 cm (74.8%), and 25 of 41 tumors greater than 0.5 cm (60.9%) disappeared up to 12 months following RFA. The trends in volume change are shown in Figure 3. The tumor volumes increased immediately after RFA because of the included safety margin and then gradually decreased. At 3 years, the mean VRR was approximately 100%, which means that the treated tumors remained as small scar-like lesions. All 54 tumors with more than 4 years of follow-up showed complete disappearance. Up until the last follow-up, 13 of the 152 PTMCs had not completely disappeared. No tumor showed regrowth of the residual ablated lesion during the follow-up period among the 13 tumors. Most of these 13 lesions had a relatively short follow-up duration (7 were followed up for 6 months, 3 for 12 months, 2 for 15 months, and 1 for 24 months).
The overall complication rate was 3.0% per patient (4/133), with the rate of major complications being 0.8% per patient (1/133). Detailed characteristics of the complications are given in Table 3. No patient experienced a life-threatening complication during the follow-up period, and all patients had normal thyroid function following RFA. None of the patients showed local tumor recurrence, cervical LN metastasis, or distant metastasis during the follow-up period. No patients needed additional surgery because of primary cancer.
This large-population study demonstrated that 139 of the 152 tumors (91.4%) completely disappeared over the long term follow-up period, with a mean duration of 39 months. During the follow-up period, there were no local tumor recurrences and no LN or distant metastases, even in the 54 patients with a follow-up period of over 4 years. No patients were referred for surgery. We found acceptable overall complication rates of 3% and 0.8% for major complications. Therefore, we believe that RFA can be useful in local tumor control for low-risk PTMCs.
Recently, several ablation techniques such as RFA, LA, and MWA have been used for treating low-risk PTMCs (456789). Among the studies that have evaluated these techniques, there were no local tumor recurrences and no distant metastases. However, in one study that enrolled 64 patients with a follow-up period of 26 months, one patient with LN metastasis was reported (8). Zhang et al. (5) included the largest population, of 92 patients, so far reported for a prospective study. They achieved a mean volume reduction of 96% at 1 year, and a complete disappearance rate of 10.2% at 7.8 months of follow-up after RFA. Although Kim et al. (6) reported a multicenter study with the longest follow-up period of 48.5 months, they enrolled only six patients. Their results showed a mean VRR of 98.5% and a complete disappearance rate of 66.7% (6). Compared with these previous studies, this study enrolled a larger population and achieved 100% complete disappearance in 54 patients after 4 years. Furthermore, the local tumor control ability of thermal ablations for recurrent thyroid cancers has been well documented (9242526).
Our study showed a 3% overall complication rate and a 0.8% rate of major complications. This is similar to those found in a meta-analysis, in which the overall complication rate was 2.4%, and the rate of major complications was 1.4% (27). In studies that evaluated primary PTMCs treated by ablation, eight patients were reported to have voice change as a complication (45). The complication rate for PTMC is relatively low compared to that for recurrent thyroid cancers, and nerve injury is limited to the recurrent laryngeal nerve (92829). As reported in several studies, including this study, we believe RFA presents favorable treatment outcomes, with no local tumor recurrences and no distant metastases.
Although the 2015 ATA guidelines suggested active surveillance for the first-line management of low-risk PTMC, a debate continues regarding this treatment option for primary low-risk PTMC. Although active surveillance can reduce overtreatment and unnecessary surgery in patients with PTMC, especially in those with high surgical risk or in those who refuse to undergo surgery (1), patients may still be concerned regarding the risks of the indwelling cancer and could suffer considerable anxiety during follow-up. Oda et al. (30) reported that 2.3% of patients showed tumor enlargement of more than 3 mm, and 0.5% had nodal metastases during active surveillance. Indeed, one study demonstrated that 6.8% of PTMC patients progressed to clinical disease during active surveillance, with the incidence being higher in the younger population, with a rate up to 22.5% (31). This means that 6.8% of the total population or 22.5% of the young population with PTMCs are inevitably potential candidates for surgery during the follow-up period. In a recent Korean multicenter active surveillance study enrolling 370 patients with PTMC, tumor progression was defined by a 50% volume change, rather than a 3 mm diameter increase. Twenty-three percent of patients experienced a tumor volume increase, and 17 patients (4.6%) had LN metastasis on final surgical pathology over a median 32.5 months of active surveillance period (32). However, our results showed no local tumor recurrence, no cervical LN metastasis, and no distant metastasis in patients with PTMCs treated by RFA, even in 54 patients with a follow-up of more than 4 years. Therefore, we cautiously suggest RFA as a reinforcement treatment option for PTMCs, as it can significantly extend the subclinical period compared to active surveillance.
Despite our positive results, debate continues regarding the real value of ablations for low-risk PTMCs. Indeed, Valcavi et al. (33) reported surgical results followed by LA for PTMCs in three patients. The postoperative histopathology revealed multiple tumor microfoci within the thyroid gland and microscopic metastases in the central neck LNs. Moreover, Ma et al. (34) recently reported results from 12 patients who underwent thermal ablation for papillary thyroid carcinoma followed by surgery. Residual cancers and LN metastasis were confirmed in the histopathology in all ablated cancers. We believe that those results might be related to a different patient population. Previous studies enrolled patients with non-low-risk PTMC (more than 1 cm, capsule invasion, and LN metastasis), while we only included low-risk PTMC patients and tried to create a sufficient ablation margin. Another difference in our study is the pretreatment imaging workup. Some studies used a contrast-enhanced US to detect the untreated area during ablation (35), while we performed both a US and contrast-enhanced CT to evaluate for LN metastasis before treatment (3637). Therefore, we suggest that RFA for primary thyroid cancer should be performed only in low-risk PTMCs and that it should be performed by an expert operator. Furthermore, careful pre-treatment evaluation using both US and contrast-enhanced CT should be performed to rule out LN metastasis.
This retrospective study has several limitations. First, there might be selection bias due to the retrospective study design. Therefore, a multicenter prospective study is needed to confirm our results even though our study has included the largest study population to date. Second, although this study included small PTMCs less than 5 mm, which were treated mostly in the early study period, the optimal low limit of tumor size for RFA treatment remains controversial. However, we are suggesting RFA as a reinforcement treatment option for active surveillance in PTMCs, and recommend this discussion on inclusion criteria and consensus to avoid overtreatment be further investigated. Lastly, the presence of extrathyroidal extension was assessed only by imaging, and not by histopathology.
In conclusion, RFA is an effective and safe treatment option for low-risk PTMCs in patients who have high surgical risk or who refuse to undergo surgery.
References
1. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016; 26:1–133. PMID: 26462967.

2. Hay ID, Thompson GB, Grant CS, Bergstralh EJ, Dvorak CE, Gorman CA, et al. Papillary thyroid carcinoma managed at the Mayo Clinic during six decades (1940–1999): temporal trends in initial therapy and long-term outcome in 2444 consecutively treated patients. World J Surg. 2002; 26:879–885. PMID: 12016468.

3. Iñiguez-Ariza NM, Brito JP. Management of low-risk papillary thyroid cancer. Endocrinol Metab (Seoul). 2018; 33:185–194. PMID: 29947175.

4. Yue W, Wang S, Yu S, Wang B. Ultrasound-guided percutaneous microwave ablation of solitary T1N0M0 papillary thyroid microcarcinoma: initial experience. Int J Hyperthermia. 2014; 30:150–157. PMID: 24571178.

5. Zhang M, Luo Y, Zhang Y, Tang J. Efficacy and safety of ultrasound-guided radiofrequency ablation for treating low-risk papillary thyroid microcarcinoma: a prospective study. Thyroid. 2016; 26:1581–1587. PMID: 27445090.

6. Kim JH, Baek JH, Sung JY, Min HS, Kim KW, Hah JH, et al. Radiofrequency ablation of low-risk small papillary thyroidcarcinoma: preliminary results for patients ineligible for surgery. Int J Hyperthermia. 2017; 33:212–219. PMID: 27590679.

7. Zhou W, Jiang S, Zhan W, Zhou J, Xu S, Zhang L. Ultrasound-guided percutaneous laser ablation of unifocal T1N0M0 papillary thyroid microcarcinoma: preliminary results. Eur Radiol. 2017; 27:2934–2940. PMID: 27853812.

8. Zhang L, Zhou W, Zhan W, Peng Y, Jiang S, Xu S. Percutaneous laser ablation of unifocal papillary thyroid microcarcinoma: utility of conventional ultrasound and contrast-enhanced ultrasound in assessing local therapeutic response. World J Surg. 2018; 42:2476–2484. PMID: 29488064.

9. Jeong SY, Baek JH, Choi YJ, Chung SR, Sung TY, Kim WG, et al. Radiofrequency ablation of primary thyroid carcinoma: efficacy according to the types of thyroid carcinoma. Int J Hyperthermia. 2018; 34:611–616. PMID: 29322881.

10. Na DG, Baek JH, Jung SL, Kim JH, Sung JY, Kim KS, et al. Korean Society of Thyroid Radiology (KSThR) and Korean Society of Radiology. Core needle biopsy of the thyroid: 2016 consensus statement and recommendations from Korean Society of Thyroid Radiology. Korean J Radiol. 2017; 18:217–237. PMID: 28096731.

11. Ha EJ, Lim HK, Yoon JH, Baek JH, Do KH, Choi M, et al. Korean Society of Thyroid Radiology (KSThR) and Korean Society of Radiology. Primary imaging test and appropriate biopsy methods for thyroid nodules: guidelines by Korean Society of Radiology and National Evidence-Based Healthcare Collaborating Agency. Korean J Radiol. 2018; 19:623–631. PMID: 29962869.

12. Tuttle RM, Haugen B, Perrier ND. Updated American Joint Committee on Cancer/tumor-node-metastasis staging system for differentiated and anaplastic thyroid cancer (eighth edition): what changed and why? Thyroid. 2017; 27:751–756. PMID: 28463585.

13. Kim HJ. Updated guidelines on the preoperative staging of thyroid cancer. Ultrasonography. 2017; 36:292–299. PMID: 28607324.

14. Shin JH, Baek JH, Chung J, Ha EJ, Kim JH, Lee YH, et al. Korean Society of Thyroid Radiology (KSThR) and Korean Society of Radiology. Ultrasonography diagnosis and imaging-based management of thyroid nodules: revised Korean Society of Thyroid Radiology consensus statement and recommendations. Korean J Radiol. 2016; 17:370–395. PMID: 27134526.

15. Jung SL, Baek JH, Lee JH, Shong YK, Sung JY, Kim KS, et al. Efficacy and safety of radiofrequency ablation for benign thyroid nodules: a prospective multicenter study. Korean J Radiol. 2018; 19:167–174. PMID: 29354014.

16. Baek JH, Kim YS, Sung JY, Choi H, Lee JH. Locoregional control of metastatic well-differentiated thyroid cancer by ultrasound-guided radiofrequency ablation. AJR Am J Roentgenol. 2011; 197:W331–W336. PMID: 21785061.

17. Kim JH, Baek JH, Lim HK, Ahn HS, Baek SM, Choi YJ, et al. Guideline committee for the Korean Society of Thyroid Radiology (KSThR) and Korean Society of Radiology. 2017 thyroid radiofrequency ablation guideline: Korean Society of Thyroid Radiology. Korean J Radiol. 2018; 19:632–655. PMID: 29962870.

18. Na DG, Lee JH, Jung SL, Kim JH, Sung JY, Shin JH, et al. Korean Society of Thyroid Radiology (KSThR). Korean Society of Radiology. Radiofrequency ablation of benign thyroid nodules and recurrent thyroid cancers: consensus statement and recommendations. Korean J Radiol. 2012; 13:117–125. PMID: 22438678.

19. Baek JH, Kim YS, Lee D, Huh JY, Lee JH. Benign predominantly solid thyroid nodules: prospective study of efficacy of sonographically guided radiofrequency ablation versus control condition. AJR Am J Roentgenol. 2010; 194:1137–1142. PMID: 20308523.

20. Jeong WK, Baek JH, Rhim H, Kim YS, Kwak MS, Jeong HJ, et al. Radiofrequency ablation of benign thyroid nodules: safety and imaging follow-up in 236 patients. Eur Radiol. 2008; 18:1244–1250. PMID: 18286289.

21. Park HS, Baek JH, Park AW, Chung SR, Choi YJ, Lee JH. Thyroid radiofrequency ablation: updates on innovative devices and techniques. Korean J Radiol. 2017; 18:615–623. PMID: 28670156.

22. Ahmed M, Solbiati L, Brace CL, Breen DJ, Callstrom MR, Charboneau JW, et al. International Working Group on Image-Guided Tumor Ablation. Interventional Oncology Sans Frontières Expert Panel. Technology Assessment Committee of the Society of Interventional Radiology. Standard of Practice Committee of the Cardiovascular and Interventional Radiological Society of Europe. Image-guided tumor ablation: standardization of terminology and reporting criteria--a 10-year update. J Vasc Interv Radiol. 2014; 25:1691–1705.e4. PMID: 25442132.

23. Baek JH, Lee JH, Sung JY, Bae JI, Kim KT, Sim J, et al. Korean Society of Thyroid Radiology. Complications encountered in the treatment of benign thyroid nodules with US-guided radiofrequency ablation: a multicenter study. Radiology. 2012; 262:335–342. PMID: 21998044.

24. Mauri G, Cova L, Ierace T, Baroli A, Di Mauro E, Pacella CM, et al. Treatment of metastatic lymph nodes in the neck from papillary thyroid carcinoma with percutaneous laser ablation. Cardiovasc Intervent Radiol. 2016; 39:1023–1030. PMID: 26911732.

25. Persichetti A, Bizzarri G, Guglielmi R, Barnabei A, Bianchini A, Coccaro C, et al. Ultrasound-guided laser ablation for local control of neck recurrences of medullary thyroid cancer. A feasibility study. Int J Hyperthermia. 2018; 35:480–492. PMID: 30204004.

26. Mazzeo S, Cervelli R, Elisei R, Tarantini G, Cappelli C, Molinaro E, et al. mRECIST criteria to assess recurrent thyroid carcinoma treatment response after radiofrequency ablation: a prospective study. J Endocrinol Invest. 2018; 41:1389–1399. PMID: 29687416.

27. Suh CH, Baek JH, Choi YJ, Lee JH. Efficacy and safety of radiofrequency and ethanol ablation for treating locally recurrent thyroid cancer: a systematic review and meta-analysis. Thyroid. 2016; 26:420–428. PMID: 26782174.

28. Kim C, Lee JH, Choi YJ, Kim WB, Sung TY, Baek JH. Complications encountered in ultrasonography-guided radiofrequency ablation of benign thyroid nodules and recurrent thyroid cancers. Eur Radiol. 2017; 27:3128–3137. PMID: 27975148.

29. Chung SR, Suh CH, Baek JH, Park HS, Choi YJ, Lee JH. Safety of radiofrequency ablation of benign thyroid nodules and recurrent thyroid cancers: a systematic review and meta-analysis. Int J Hyperthermia. 2017; 33:920–930. PMID: 28565997.

30. Oda H, Miyauchi A, Ito Y, Yoshioka K, Nakayama A, Sasai H, et al. Incidences of unfavorable events in the management of low-risk papillary microcarcinoma of the thyroid by active surveillance versus immediate surgery. Thyroid. 2016; 26:150–155. PMID: 26426735.

31. Ito Y, Miyauchi A, Kihara M, Higashiyama T, Kobayashi K, Miya A. Patient age is significantly related to the progression of papillary microcarcinoma of the thyroid under observation. Thyroid. 2014; 24:27–34. PMID: 24001104.

32. Oh HS, Ha J, Kim HI, Kim TH, Kim WG, Lim DJ, et al. Active surveillance of low-risk papillary thyroid microcarcinoma: a multi-center cohort study in Korea. Thyroid. 2018; 28:1587–1594. PMID: 30226447.

33. Valcavi R, Piana S, Bortolan GS, Lai R, Barbieri V, Negro R. Ultrasound-guided percutaneous laser ablation of papillary thyroid microcarcinoma: a feasibility study on three cases with pathological and immunohistochemical evaluation. Thyroid. 2013; 23:1578–1582. PMID: 23978269.

34. Ma B, Wei W, Xu W, Wang Y, Guan H, Fan J, et al. Surgical confirmation of incomplete treatment for primary papillary thyroid carcinoma by percutaneous thermal ablation: a retrospective case review and literature review. Thyroid. 2018; 28:1134–1142. PMID: 29962285.

35. Zhan J, Ding H. Application of contrast-enhanced ultrasound for evaluation of thyroid nodules. Ultrasonography. 2018; 37:288–297. PMID: 30213158.

36. Suh CH, Baek JH, Choi YJ, Lee JH. Performance of CT in the preoperative diagnosis of cervical lymph node metastasis in patients with papillary thyroid cancer: a systematic review and meta-analysis. AJNR Am J Neuroradiol. 2017; 38:154–161. PMID: 27789450.

37. Lee Y, Kim JH, Baek JH, Jung SL, Park SW, Kim J, et al. Value of CT added to ultrasonography for the diagnosis of lymph node metastasis in patients with thyroid cancer. Head Neck. 2018; 40:2137–2148. PMID: 29756249.

Fig. 1
Flow chart of patient enrollment.
M = months, PTMC = papillary thyroid microcarcinoma, RFA = radiofrequency ablation, US = ultrasonography
Fig. 2
RFA example for PTMC.
A. Proven hypoechoic PTMC is located on right side of isthmus with spiculated margin. B. Lidocaine for local anesthesia at puncture site and around thyroid capsule was injected (thin arrows), and hydro dissection technique was performed due to tumor being located close to trachea (thick arrows). C. This was followed by ablation with thyroid-dedicated electrode. D. Echogenicity changed in ablated zone, displaying large ablation zone, including primary cancer and surrounding normal thyroid tissue.
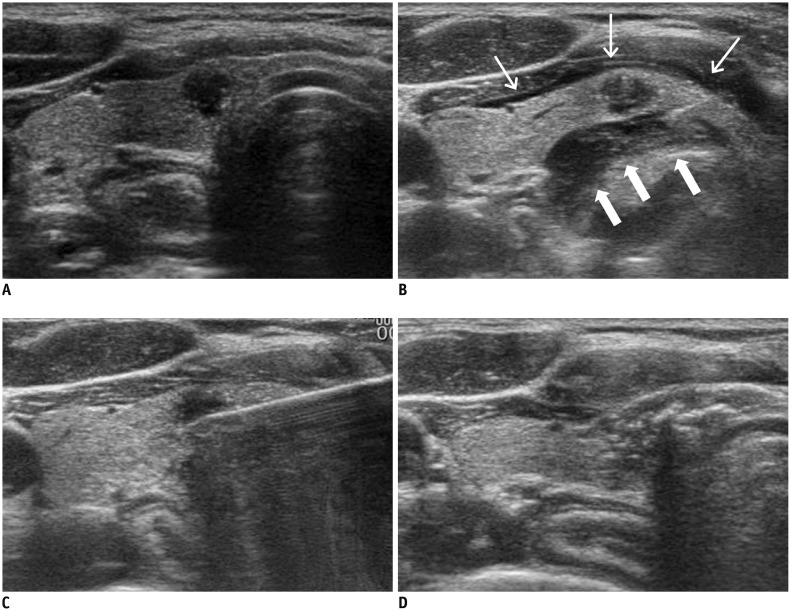
Fig. 3
Serial mean volume reduction on ultrasonography.
Tumor volumes increased immediately after RFA and then gradually decreased. Mean volume reduction rate reached 100% at 36 M of F/U for total tumors (at 24 M of F/U for tumors ≥ 0.5 cm; at 36 M of F/U for tumors < 0.5 cm). Interval between from −220 to −900 were spaced. W = week
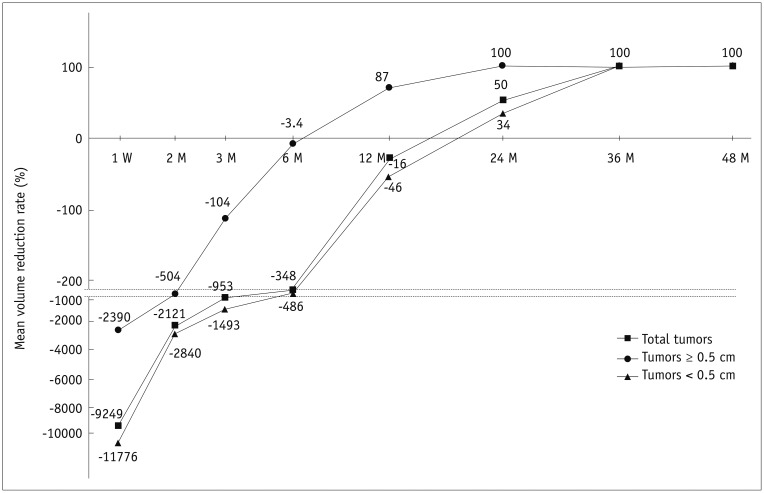
Table 1
Demographics and Characteristics of PTMCs and RFA
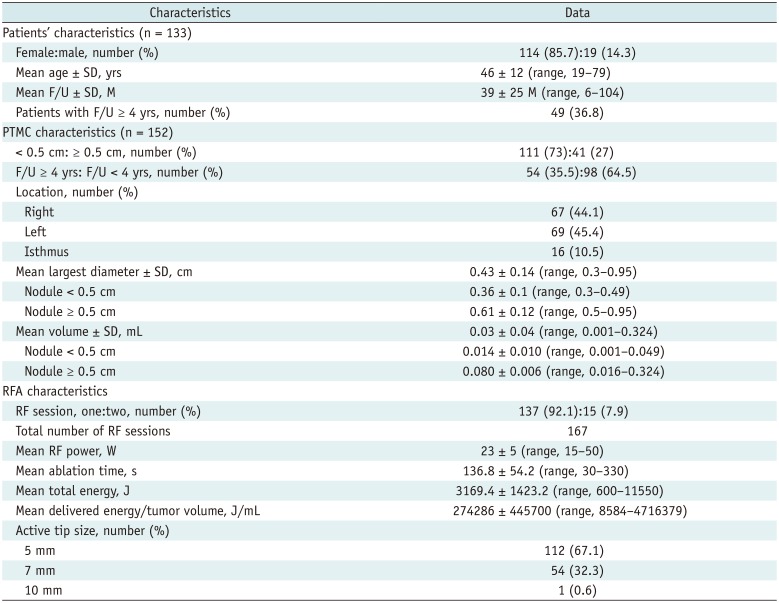
Table 2
Treatment Responses after RFA

Table 3
Characteristics and Management of Complications
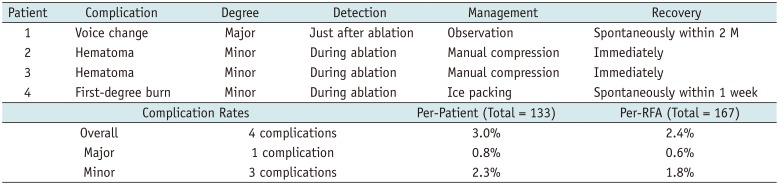
| Complication Rates | Per-Patient (Total = 133) | Per-RFA (Total = 167) | |||
|---|---|---|---|---|---|
| Overall | 4 complications | 3.0% | 2.4% | ||
| Major | 1 complication | 0.8% | 0.6% | ||
| Minor | 3 complications | 2.3% | 1.8% | ||




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download