Abstract
Pulmonary invasive mucinous adenocarcinoma (PIMA) formerly known as mucinous bronchioalveolar carcinoma is a relatively rare variant of pulmonary adenocarcinoma, but the incidence of this tumor is known to be rising. Imaging findings may include nodules, masses and lobar involvement of variable densities, but the majority present as air-space consolidation. We report a case of PIMA with radiological-pathological correlation in a 23-year-old male which presented as a large cavitary mass mimicking congenital cystic lung disease.
Pulmonary invasive mucinous adenocarcinoma (PIMA) is a variant of invasive adenocarcinoma of the lung (1). The incidence of this tumor is known to be rising (2). The tumor cells demonstrate goblet cell histology with copious intracytoplasmic mucin material (1, 2). Imaging may show a broad spectrum of findings, but lobar or segmental consolidation is found in the majority (1, 3-5). This case presented as a large mass-like consolidation with multiple internal air-cysts and/or pseudo-cavitations which mimicked complicated congenital cystic lung disease in a patient with relatively young age to consider lung cancer. Therefore, we report a case of PIMA presenting as a large cavitary mass in a 23-year-old male with clinical and radiological findings, and a review of the literature.
A 23-year-old male visited our hospital for further evaluation of abnormal findings on a chest radiograph. He had a previous medical history of 6-months treatment for pulmonary tuberculosis with complete improvement 10 years ago. The initial chest radiogram showed a 6 × 5 cm irregular mass with suspicious cavitation in the right lower lobe. Contrast-enhanced chest computed tomography (CT) showed a 6 × 5 cm mass abutting the right major fissure in the right anterolateral basal lung. The mass had multiple air-cysts with a size range of 2 mm to 25 mm, resulting in a cavitary appearance. An internal air bronchogram, CT angiogram sign and pleural tagging were also present in the mass which had two adjacent satellite nodules (Fig. 1A-C). There was no pleural or pericardial effusion, nor detectable mediastinal or hilar lymphadenopathy. The initial differential diagnosis was an inflammatory lesion associated with congenital cystic adenomatoid malformation (CCAM) or a pneumonic malignancy such as adenocarcinoma. No endobronchial lesion was found on the bronchoscopic exam. The results of the bronchial washing and transbronchial lung biopsy were also non-specific.
A follow-up chest CT 2 months later showed no interval change of the mass. Follow-up 18F-fluorodeoxyglucose (FDG) positron emission tomography-CT showed mild FDG uptake (standardized uptake value = 1.9) by the lesion without evidence of an extrathoracic malignancy or metastasis (Fig. 1D). With the suspicion of a low FDG-avid malignancy, the patient underwent video-assisted thoracoscopic surgery lobectomy. Intraoperative findings showed a large gleamy mass. Incision of the mass demonstrated entire mucinous change of the mass with abundant foamy secretions. The intraoperative frozen biopsy result suggested a benign cystic lesion such as a mucinous cystadenoma, but the final pathologic diagnosis confirmed the mass as a mucinous adenocarcinoma (Fig. 1E, F). The patient underwent four rounds of paclitaxel and cisplatin chemotherapy. On a follow-up CT, a new small nodule appeared in the rearranged right upper lobe (RUL), which showed a gradual increase on the subsequent follow-up CT images. The patient had wedge resection of the RUL nodule. The pathologic diagnosis confirmed primary or metastatic mucinous adenocarcinoma. Immunohistochemical stains showed negative thyroid transcription factor 1 and cytokeratin 20 (CK20) with positive CK7. Polymerase chain reaction test for genotype evaluation revealed negative epidermal growth factor receptor (EGFR) mutation.
PIMA is classified as a subtype of variants of invasive adenocarcinoma by the International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society for lung adenocarcinoma on 2011 (1). It was formerly known as mucinous bronchioalveolar carcinoma (BAC) according to the 2004 World Health Oraganization classification (6). However, the use of the term BAC overlaps several types of entities including adenocarcinoma in situ (AIS), minimally invasive adenocarcinoma (MIA), lepidic predominant adenocarcinoma (LPA), mixed subtype adenocarcinoma and invasive mucinous adenocarcinoma, causing confusion (1). The new classification puts invasive mucinous adenocarcinomas and formerly nonmucinous BAC into different categories, due to their distinct characteristics in terms of their genetic, histologic and imaging features (1). Invasive mucinous adenocarcinoma may show K-ras mutation frequently, but lacks EGFR mutation which is more likely to show in nonmucinous adenocarcinomas (1, 7). Pathologically, invasive adenocarcinoma has the distinguishing feature of goblet cell histology with copious intracytoplasmic mucin material (1, 2).
There is a slightly higher incidence among women, non-smokers and people with a younger age at presentation compared to other types of lung cancer (1, 2). Patients may be asymptomatic or show non-specific symptoms such as dyspnea, cough, sputum, and hemoptysis (2). Our patient was young, and a non-smoker without any presenting symptoms. Also the tumor showed only mild uptake on the FDG positron emission tomography (PET)-CT, so a congenital cystic lung disease such as complicated CCAM was considered as the diagnosis of choice. However, there were several imaging findings and clinical features suggesting a malignancy such as PIMA. The imaging findings of PIMA may be variable, but the majority show segmental or lobar consolidation with an air bronchogram and the CT angiogram sign. A focal mass or the nodular type is less common. The density of these lesions is variable, ranging from ground glass opacity to a part-solid or solid density. An internal air bronchogram, cyst and/or pseudocavitation are characteristic findings. Multifocal and multilobar involvement is also common (1, 3-5). Our case showed the manifestation of a single cavitary mass with a large cavity size, which was an unusual feature, compared to prior reports (3-5, 8). However, the patent internal air-bronchogram of the mass suggested the possibility of a consolidative lung malignancy and not a dysplastic lung. Also, the lobulated shape and spiculated margin of the tumor with pleural tags and daughter nodules were suggestive of a malignant condition. A mucin-producing tumor such as a mucinous adenocarcinoma may develop large pseudocavitation (2). Also, it shows relatively subtle enhancement after contrast enhancement, resulting in the CT angiogram sign, as in our case (2). The abundant mucin component of the tumor causes only a mild uptake on the FDG PET-CT (9, 10).
Adenocarcinoma without EGFR mutation is known to have a worse prognosis and the majority of PIMAs are known to lack EGFR mutation (7). Likewise, PIMA is an aggressive tumor with a much poorer prognosis compared to AIS, MIA, and LPA (1). Our patient developed metastatic or multicentric PIMA on follow-up after the surgical excision, indicating a poor prognosis. Therefore, early diagnosis and surgical excision are essential in this malignancy. A radiologist should be familiar with several characteristic imaging findings of this disease to make an early non-invasive diagnosis.
In conclusion, this case presented a rare manifestation of PIMA in a very young patient with a single large cavitary mass. However, several characteristic imaging features were suggestive of this rare tumor, which can show unusual radiological and clinical characteristics compared with other primary lung cancers. If a radiologist is familiar with such imaging findings and is aware that PIMA is a low FDG-avid tumor, PIMA may be able to be included in the differential diagnosis when such imaging findings are present.
Figures and Tables
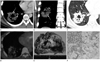
Fig. 1
A 23-year-old male with pulmonary invasive mucinous adenocarcinoma.
A-C. Contrast enhanced mediastinal (A. axial image, B. coronal image) and lung window (C) setting chest CT shows a cavitary mass with internal air bronchogram (white arrows in C) and pseudo-cavitation (arrowhead in C), CT angiogram sign (white arrows in A, B) and surrounding daughter nodules (black arrows in C) in right lower lobe.
D. FDG PET-CT scan shows mild FDG uptake (SUV = 1.9) of the mass.
E. Photograph of the gross specimen demonstrates lobulated mass containing cystic lesion filled with gelatinous substance.
F. On microscopy, the tumor cells arrange along alveolar septa (H&E, × 20). The tumor cells have a goblet cell appearance with copious intracytoplasmic mucin (Inset, H&E, × 100).
Note.-FDG = 18F-fluorodeoxyglucose, PET = positron emission tomography, SUV = standardized uptake value
References
1. Travis WD, Brambilla E, Noguchi M, Nicholson AG, Geisinger KR, Yatabe Y, et al. International association for the study of lung cancer/american thoracic society/european respiratory society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol. 2011; 6:244–285.
2. Lee KS, Kim Y, Han J, Ko EJ, Park CK, Primack SL. Bronchioloalveolar carcinoma: clinical, histopathologic, and radiologic findings. Radiographics. 1997; 17:1345–1357.
3. Austin JH, Garg K, Aberle D, Yankelevitz D, Kuriyama K, Lee HJ, et al. Radiologic implications of the 2011 classification of adenocarcinoma of the lung. Radiology. 2013; 266:62–71.
4. Akira M, Atagi S, Kawahara M, Iuchi K, Johkoh T. High-resolution CT findings of diffuse bronchioloalveolar carcinoma in 38 patients. AJR Am J Roentgenol. 1999; 173:1623–1629.
5. Patsios D, Roberts HC, Paul NS, Chung T, Herman SJ, Pereira A, et al. Pictorial review of the many faces of bronchioloalveolar cell carcinoma. Br J Radiol. 2007; 80:1015–1023.
6. Beasley MB, Brambilla E, Travis WD. The 2004 World Health Organization classification of lung tumors. Semin Roentgenol. 2005; 40:90–97.
7. Finberg KE, Sequist LV, Joshi VA, Muzikansky A, Miller JM, Han M, et al. Mucinous differentiation correlates with absence of EGFR mutation and presence of KRAS mutation in lung adenocarcinomas with bronchioloalveolar features. J Mol Diagn. 2007; 9:320–326.
8. Ioachimescu OC, Mehta AC. From cystic pulmonary airway malformation, to bronchioloalveolar carcinoma and adenocarcinoma of the lung. Eur Respir J. 2005; 26:1181–1187.
9. Berger KL, Nicholson SA, Dehdashti F, Siegel BA. FDG PET evaluation of mucinous neoplasms: correlation of FDG uptake with histopathologic features. AJR Am J Roentgenol. 2000; 174:1005–1008.
10. Cook GJ, Wegner EA, Fogelman I. Pitfalls and artifacts in 18FDG PET and PET/CT oncologic imaging. Semin Nucl Med. 2004; 34:122–133.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download