Abstract
Figures and Tables
Fig. 1
Representative photomicrographs of rat lungs on day 14 (A). H&E staining; magnification, 100 ×. Scale bar indicates 200 µm. Morphometric data of rat lungs on day 14 are shown (B, C). n = 5-8 per group. *P <0.05. BPD, bronchopulmonary dysplasia; V, vehicle; DFX, deferoxamine.

Fig. 2
Representative photomicrographs illustrating the results of immunohistochemical analysis of PECAM-1 in rat lungs on day 14 (A). Arrows indicate pulmonary vessels stained with PECAM-1. Magnification, 400 ×. Scale bar indicates 50 µm. The number of pulmonary vessels per high-power field (HPF) (B) and pulmonary vascular density (C) are displayed in a bar graph. n = 5-8 per group. *P < 0.05. PECAM, platelet endothelial cell adhesion molecule; BPD, bronchopulmonary dysplasia; V, vehicle; DFX, deferoxamine.

Fig. 3
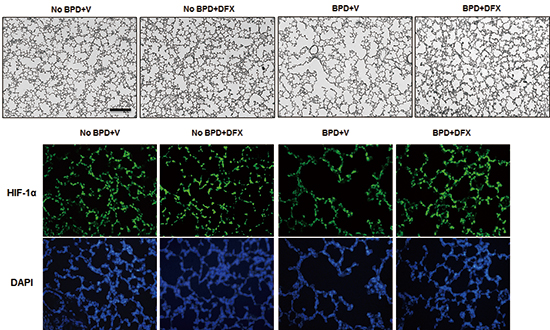
Representative photomicrographs illustrating the results of immunofluorescence analysis of HIF-1α in rat lung on day 14 (A). Treatment with DFX recovered the expression of HIF-1α in the lungs of BPD rats. Tissue sections were serially cut at 4 µm and subjected to immunofluorescence staining of HIF-1α. Lung tissues in the four experimental groups were homogenized, and 20 µg of protein was subjected to Western blotting using an anti-HIF-1α antibody (B). Tubulin was used as a loading control. DFX, deferoxamine; HIF, hypoxia-inducible factor; BPD, bronchopulmonary dysplasia; V, vehicle; DAPI, 4',6-diamidino-2-phenylindole.
Fig. 4
Deferoxamine induced HIF-1α in a dose-dependent manner in human small airway epithelial cells (HSAEpCs) (A). HSAEpCs were incubated with 10, 20, 30 or 65 µM DFX for 8 hr, and HIF-1α expression was evaluated by Western blotting. HSAEpCs were incubated with 65 µM DFX for the indicated times (B). The expression of HIF-1α was analyzed by Western blotting. DFX, deferoxamine; HIF, hypoxia-inducible factor.

Fig. 5
Deferoxamine-induced HIF-1α was active. Deferoxamine activated VEGF promoter (A). Human small airway epithelial cells (HSAEpCs) were co-transfected with 1 µg of VEGF promoter-luciferase plasmid and β-galactosidase plasmid. After 48 hr, the cells were treated with 10 or 65 µM DFX for 16 hr and lysed for the luciferase assay. Luciferase activity was normalized to β-galactosidase activity. Bars represent the mean±SD (n = 4) of luciferase activity. Deferoxamine induces the mRNA expression of HIF-1α target genes (B, C). Cells were treated with 65 µM DFX for the indicated times and subsequently harvested. Total RNAs were extracted from the cells, and the mRNA levels of CA9 (B), LOX (C), and 18S were analyzed by RT-qPCR. Each bar represents the mean±SD of four separate experiments. *P < 0.05. DFX, deferoxamine; HIF, hypoxia-inducible factor.

Notes
Funding This work was supported by the SNUBH Research Fund (grant No. 03-2007-007) and by Basic Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2010-0021644).
AUTHOR CONTRIBUTION Study conception and design: Choi CW, Chun YS, Kim BI. Animal experiments: Choi CW, Lee HJ, Park HS. Acquisition of data: Choi CW, Lee HJ, Park HS, Chun YS. Data analyses: Choi CW, Chun YS, Kim BI. Drafting and writing the manuscript: Choi CW, Lee J, Park HS, Chun YS, Kim BI. Final manuscript approval: all authors.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download