Artificial intelligence (AI) is defined as any techniques that enable the computer to mimic the human brain to simulate human intelligence process. A branch of AI technology is Machine Learning (ML) which is a study of an algorithm to build a model of sample data to make prediction or decision without being programmed for the task. Deep Learning (DL) is a class of ML method based on an artificial neural network utilizing information processing and distribute communication to extract higher-level feature from raw input data. DL can outperform conventional ML as they adapt, learn, and continue to improve with more data, particularly heterogeneous ones such as healthcare data.
There are several DL classes. Computer vision known as convolutional neural network (CNN) applied for data with natural spatial invariance such as an image. CNN can aid flagging and second opinion in pathology, ophthalmology, etc. CNN needs extensive data and labeled dataset for training. Natural Language Processing (NLP) also recurrent neural network (RNN) is effective at processing sequential input such as language, speech, and time-series data therefore particularly suitable for electronic health record (EHR) data. RNN can translate patientcare provider conversation, predict future medical incidents from EHR. Reinforcement Learning (RL) is DL for training to interact with the environment through trial and error, demonstration, or a hybrid of both. RL can be applied to robotic surgery with computer vision models. Generalized Deep Learning can adapt to nuanced data requiring specialized treatment such as genomic data which can be applied to predict pathogenicity and phenotype from genome data.[1]
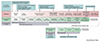
AI is applied in drug discovery and development such as validation of target,[2] designing a new drug, drug repurposing, designing polypharmacological agents, improving R&D efficiency through trial design optimization, selection of patient population and monitoring efficacy and safety of patients in clinical trials (Fig. 1). In drug discovery, if AI is properly trained with optimal data, it can provide insight into a new molecule to target, complex metabolic pathways of a compound, and predict toxicity. For example, the US federal government's Tox21 program, a collaboration among the Environmental Protection Agency, the National Institutes of Health, and the Food and Drug Administration, maintains an extensive data set of molecules and their toxicity against key human proteins.[3] This data set can be fed to AI to digest in search of patterns of association between structure, properties, function, and possible toxic effects. The generative adversarial network is one application to develop novel small-molecule compounds to treat various disorders. Another platform aims to mimic the decision making of a medicinal chemist while also learning from human medicinal chemists' input.[3] Currently there is no reported success case of AI-driven new drug discovery, but numerous partnership between the pharma and AI companies will have a successful outcome in a few years, and this will change the industry permanently.
In clinical development, AI tools such as NLP and computer vision can combine omics data, EHR, and biomarkers to identify and characterize most appropriate subpopulation for a trial. This will reduce population heterogeneity and enrich the population. Prognostic enrichment is used for neurological diseases such as Alzheimer disease (AD) where key biomarkers were replaced by a combination of multiple cheaper non-invasive measures applying ML methods. Complex models such as disease progression models are required for predictive enrichment. In AD, clinical trial simulation tools were successfully developed.[4]
Patient monitoring can improve when AI and wearable technology are combined. AI can also be used to predict the risk of patient dropout from EHR or behavior dynamically. Other technologies being combined with AI are the internet of things (IoT) and blockchain. The AI in clinical development will eventually enable efficient patient selection and timely recruitment which is a critical bottleneck in drug development.[4]
Clinical pharmacology discipline has always emphasized model-based drug development (MBDD). The quantitative pharmacology or MBDD will have wider application and power when AI tools are incorporated. AI application for drug discovery and development also needs domain knowledge of clinical pharmacologist such as ADMET, PK/PD, and dose-response analysis and so on for realistic performance in lead optimization, pharmacology prediction, toxicology prediction, and clinical trial design from phase 1 to 3. The AI drug discovery process will have better performance with collaboration with domain experts who have insight into target product profile (TPP) and label driven drug development.
Figures and Tables
Figure 1
Utilisation of artificial intelligence (AI) in the drug development process. The outcomes and strategies of the various components of the drug development process are described. The applications of AI at each stage of drug development are also shown.[3] (from Drug Discovery Today. Volume 24, Number 3, March 2019)
References
1. Esteva A, Robicquet A, Ramsundar B, Kuleshov V, DePristo M, Chou K, et al. A guide to deep learning in healthcare. Nat Med. 2019; 25:24–29. DOI: 10.1038/s41591-018-0316-z.

2. Bakkar N, Kovalik T, Lorenzini I, Spangler S, Lacoste A, Sponaugle K, et al. Artificial intelligence in neurodegenerative disease research: use of IBM Watson to identify additional RNA-binding proteins altered in amyotrophic lateral sclerosis. Acta Neuropathol. 2018; 135:227–247. DOI: 10.1007/s00401-017-1785-8.

3. Mak KK, Pichika MR. Artificial intelligence in drug development: present status and future prospects. Drug Discov Today. 2019; 24:773–780. DOI: 10.1016/j.drudis.2018.11.014.

4. Harrer S, Shah P, Antony B, Hu J. Artificial Intelligence for Clinical Trial Design. Trends Pharmacol Sci. 2019; 40:577–591. DOI: 10.1016/j.tips.2019.05.005.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download