Abstract
The safety and efficacy of fimasartan have been evaluated through post-marketing surveillance in real world clinical practice. The multi-center, prospective, open-label and non-interventional study. A total of 3,945 patients (3,729 patients for safety assessment and 3,473 patients for efficacy assessment) were screened in patients with essential hypertension in 89 study centers from 9 September 2010 through 8 September 2016. Among the total patients, 2,893 patients (77.6%) were administered fimasartan for 24 weeks or longer and were classified as ‘patients with long-term follow-up’, and the additional safety and efficacy analysis were performed. The improvement was defined as systolic blood pressure (SBP) controlled to ≤ 140 mmHg or decreased SBP differences ≥ 20 mmHg after treatment or diastolic blood pressure (DBP) controlled to ≤ 90 mmHg or decreased DBP differences ≥ 10 mmHg after treatment. Adverse drug reactions (ADRs) were reported in 3.8% patients; dizziness, and hypotension were the most frequently reported ADRs in total patients. The results of patients with long-term follow-up were comparable with total patients. The overall improvement rate in all efficacy assessment at the last visit was 87.1% (3,025/3,473 patients). The overall improvement rate of the patients with long-term follow-up was 88.9%. Fimasartan was well tolerated, with no new safety concerns identified and an effective treatment in the real world clinical practice for Korean patients with hypertension.
Hypertension is a risk factor of cardiovascular diseases affecting a population of more than 1 billion throughout the world and is a serious disease directly and indirectly causing the death of young adults and the middle-aged who are active in economic activities as well as the elderly. According to the study of Lawes, et al., the ‘premature death’ in 7.6 million people out of the world population and the loss of 92 million disability-adjusted life years were affected by hypertension, and 54% of stroke and 47% of ischemic heart diseases were caused by hypertension.[1] It was confirmed in Korean studies that hypertension is a factor related to stroke and transient ischemic attack or a risk factor of death from cardiovascular diseases.[234] Also, WHO predicted that the cardiovascular diseases caused by hypertension or other risk factors would become the world's number 1 cause of death by 2020.[5] Therefore, the purpose of hypertension treatment is to reduce the morbidity and mortality rates of cardiovascular diseases.
Typical antihypertensive agents include beta-blockers, renin inhibitors, angiotensin converting enzyme inhibitor (ACEI), angiotensin II receptor blocker (ARB), calcium channel blocker (CCB), diuretics, etc. These therapeutic agents are all employing the principle of blocking the physiological mechanism causing hypertension, and demonstrate antihypertensive effect. ACEIs have played an important role in the treatment of hypertension and heart failure for the past 10 years or so, but their use has been limited due to well-known side effects such as dry cough and angioedema. These side effects are believed to be caused by the inhibition of the metabolism of bradykinin and substance P in addition to the inhibition of the generation of angiotensin II by ACE inhibitors.[678] Out of two types of typical receptors of angiotensin II, ARB drugs selectively block AT1 receptor which was confirmed to mediate most of the actions by angiotensin II such as vasoconstriction and secretion of aldosterone and have less side effects of ACE inhibitors, so they are being widely used recently. It has proved to be clinically effective not only on hypertension but also on kidney diseases and heart failure of patients with type 2 diabetes.
Fimasartan, the ninth and latest ARB, was approved for the treatment of hypertension by the Ministry of Food and Drug Safety (MFDS) on September 9, 2010. Fimasartan, a pyrimidin-4(3H)-one derivative of losartan with the imidazole ring replaced, which enables higher potency and longer duration than losartan.[9]
A total of 5 clinical studies have performed for fimasartan in patients with hypertension - phase IIa and phase IIb study,[10] ambulatory blood pressure monitoring (ABPM) study,[11] phase III active-controlled study,[12] and phase IIIb study[13] - and demonstrated the clinical efficacy of fimasartan. This post-marketing surveillance data was reported to MFDS on December 7, 2016 and updated to product information on August 18, 2017. The objective of this article is to provide an evaluation of the clinical utility of fimasartan in the treatment of patients with hypertension from the results of this post-marketing surveillance.
This was multi-center, prospective, open-label and non-interventional study. The study was approved by the respective Institutional Review Boards. The data was collected across 89 centers in Korea from September 9, 2010 to September 8, 2016. The objective of the study was to evaluate the safety and efficacy of fimasartan (Kanarb®, Boryung pharmaceutical Co., Ltd) as an antihypertensive therapy during a planned treatment and observation period for at least 8 weeks.
This study enrolled all patients sequentially and thoroughly who received fimasartan and provided consent to participation in this study in outpatients or hospitalized patients who had the indication (essential hypertension) of fimasartan. However, those patients who were prohibited from fimasartan administration (patients who have hypersensitivity to fimasartan or any of the ingredients contained in fimasartan, pregnant or breast-feeding women, patients on renal dialysis, patients with moderate to severe hepatic impairment, patients with biliary atresia, patients with a genetic condition such as galactose intolerance or glucose-galactose malabsorption, patients with diabetes or moderate to severe renal impairment (GFR < 60 mL/min/1.73m2), or patients with diabetic nephropathy who are taking angiotensin-converting enzyme (ACE) inhibitors) were excluded.
Patients were to receive once daily fimasartan 30 mg to 60 mg at the same time each day if possible (e.g., in the morning) with or without having a meal and if the blood pressure was not regulated well at this dose, fimasartan was increased up to once daily 120 mg. Considering that “MFDS Notification No. 2010-94” specifies the total number of patients as 3,000, this study has set a goal to make assessment on more patients than the specified number. Although, antihypertensive agents are normally administered for an extended period of time of 24 weeks or longer, investigation on long-term use was performed in approximately 15% or more of the total patients. Patients who received the fimasartan for 24 weeks or longer were classified as patients with long-term follow-up.
This study had following items to be evaluated regarding demographic characteristics, drug administration status, safety, and efficacy.
The demographic data were collected for study center, sex, age, and BMI of patients. The date of diagnosis and duration of disease regarding medical history of hypertension and other diseases, and presence of hepatic or renal impairment were investigated. For administration conditions of fimasartan, number of daily administrations, duration of administration, treatment compliance, change in dose and reason for dose change, etc. were investigated. Efficacy assessment was conducted based on the blood pressure measured at each visit and by classifying efficacy by ‘clinical symptoms’ and ‘overall improvement rate’ at the last visit. Clinical symptoms were categorized as either ‘Improved’, ‘Worsened’, or ‘Unchanged’. Clinical symptom was assessed as ‘Improved’ if any of the followings was met: 1) postdose DBP decreased ≥ 10 mmHg from pre-dose: 2) post-dose DBP regulated to a normal blood pressure of ≤ 90 mmHg: 3) post-dose SBP decreased by ≥ 20 mmHg from pre-dose: or 4) post-dose SBP regulated to a normal blood pressure of ≤ 140 mmHg. In addition, if the blood pressure was increased after administration of fimasartan, it was defined as ‘Worsened’ while any case that fell under neither ‘Improved’ nor ‘Worsened’ was defined as ‘Unchanged’.
For the decision on the overall improvement rate at the last visit of each patient, it was assessed as ‘Improved’ if blood pressure lowering effect was observed after treatment, as ‘Unimproved’ if not included in the improved range or worsened, or as ‘Unassessible’ if cannot be evaluated compared to pre-treatment status.
The safety assessment including the incidence of any adverse events (AEs) and serious adverse events (SAEs) along with their severity and relationship to the fimasartan during or after administration.
The severity of AEs was categorized into Mild, Moderate, or Severe while the causal relationship was evaluated under the categories of Certain, Probable/Likely, Possible, Unlikely, Conditional/Unclassified, or Unassessible/Unclassifiable. ADRs defined as AEs for which causal relation to fimasartan could not be denied. All data were collected and managed by established Target eCRF® database system. Medical coding was performed for medical history, concomitant medications, and adverse events. Medical history was classified by System Organ Class (SOC) and Preferred Term (PT) of WHOART 092, 2009. Drug administration status was standardized into Anatomical class (level 1) and Therapeutic class (level 2) using WHO drug reference 2015 (ATC Code). Also, all collected AEs were standardized into SOC and PT prior to database lock using MedDRA 19.0.
The analysis set was classified as the safety analysis set and the efficacy analysis set. Case report form collection set was defined as the total patients whose case report forms were collected and the safety analysis set was defined as the patients who received at least one dose of fimasartan and had safety follow-up (phone call, letter, e-mail, request for additional visit, etc.) completed. The efficacy analysis set was defined as the patients who were included in the safety analysis set, excluding patients who received fimasartan for less than 8 weeks and patients who did not conduct efficacy assessment. Also, from the above safety analysis set and efficacy analysis set, the patients who received the fimasartan for 24 weeks or longer were classified as the patients with long-term follow-up group.
For the demographic characteristics, the descriptive statistics (number of patients, mean, standard deviation, median, minimum, maximum) were presented for continuous data, and frequencies and percentages were presented for categorical data.
In the safety assessment, the incidence and the number of AEs has been summarized. In addition, the incidence of AEs by factor was tested using the chi-square test or the fisher's exact test.
In the efficacy assessment, the number of patients (n) and percentage (%) of clinical symptom assessment (Improved, Unchanged, or Worsened) at each visit and the overall improvement rate at the last visit (Improved, Unimproved, or Unassessible) in the efficacy analysis set were described. The overall improvement rate, in particular, was classified as effective for Improved cases and as ineffective for Unimproved or Unassessible cases to perform the efficacy assessment of fimasartan by factor. All analysis was performed for demographic characteristics, safety, and efficacy equally in both all patient group and patients with long-term follow-up group using the fimasartan for 24 weeks or longer. Also, all statistical analysis were performed using SAS® Version 9.4 (SAS institute, Cary, NC, USA) with a two-sided test at a 5% significance level.
A total of 3,945 patients with essential hypertension were screened from 89 centers. Of these, 216 patients who were administered prior to informed consent, were not administered fimasartan, were prohibited from receiving fimasartan, or were lost to follow-up were excluded, and consequently 3,729 patients included in the safety assessment. Two hundred fifty six patients were excluded from the efficacy assessment due to administration for < 8 weeks or the efficacy assessment not done, and consequently 3,473 patients had had the efficacy assessment. There were a total of 2,893 patients with long-term follow-up for safety assessment who received fimasartan for 24 weeks or longer, of whom 2,842 patients were included in the efficacy assessment (Fig. 1).
Of 3,285 (88.1%) patients completed in this study, while 444 (11.9%) patients were discontinued. The reasons for discontinuation were no visit (n=159, 35.8%), adverse events (n=96, 21.6%), poor response (n=89, 20%), and others (n=100, 22.5%).
Among a total of 3,729 patients for safety assessment, 2,043 patients (54.8%) were males and 1,686 patients (45.2%) were females. The mean age of these patients was 63.8±12.1 years, and 1 patient (0.03%) was younger than 18 years and 1,177 patients (31.6%) were more than 70 years. The mean BMI was 25.0±3.3 kg/m2 and all patients were essential hypertension and the average duration of hypertension was 62.5±74.0 months. The mean SBP and DBP at the baseline were 142.5±18.1 mmHg and 83.9±13.0 mmHg, respectively. The medical histories were hepatic impairment in 46 patients (1.2%), renal impairment in 44 patients (1.2%), and others in 3138 patients (84.2%). The total administration period of fimasartan was 208.9±97.0 days in total patients and 242.7±79.3 days in the patients with long-term follow-up. Such demographic characteristics were comparable with the patients with long-term follow-up for 24 weeks or longer (Table 1).
A total of 1,043 AEs were reported in 724 patients (19.4%), and 160 AEs related to the study drug (i.e., adverse drug reaction; ADR) were reported in 142 patients (3.8%) (Table 2). Dizziness (1.3%) and hypotension (0.6%) were most frequently reported ADRs. A severity of ADRs were of mild (91.9%), moderate (8.1%), and no severe intensity. Eighty-two SAEs were reported in 71 patients (1.9%) and no reported serious ADRs.
Additionally, 643 unexpected AEs not indicated on the label were reported in 490 patients (13.1%), and 42 unexpected ADRs were reported in 35 patients (0.9%). Chest pain (0.9%) and pharyngitis (0.7%) were most frequently reported unexpected AEs and gastritis (0.2%) and blood pressure increased (0.1%) were most frequently reported unexpected ADRs.
Among a total of 2,893 patients for safety assessment for 24 weeks or longer, 803 AEs were reported in 540 patients with long-term follow-up (18.7%), and 76 ADRs were reported in 67 patients with long-term follow-up (2.3%). Dizziness (0.9%) and hypotension (0.2%) were most frequently reported ADRs. Fifty-eight SAEs were reported in 49 patients with long-term follow-up (1.7%), but no serious ADR reported. In addition, 525 unexpected AEs were reported in 392 patients with long-term follow-up (13.6%), and 27 unexpected ADRs were reported in 21 patients long-term follow-up (0.7%).
Reported ADRs has been shown by SOC/PT (Table 3) and by severity (Supplement Table 1), respectively.
Based on the differences in the incident of AEs by demographic factors, the following showed statistically significant differences; sex (p-value=0.0066), presence or absence of baseline disease (p-value<0.0001), use of concomitant medications (p-value<0.0001), the elderly (based on 70 years of age: p-value= 0.0001), patients with hepatic impairment (p-value=0.0025), and patients with renal impairment (p-value<0.0001) (Table 4). However, the differences in the incidences of ADRs regarding the factors showed no statistically significant results; sex (p-value=0.8877), age (p-value=0.0864), administration of concomitant drugs (p-value=0.1698), and patients with renal impairment (p-value=0.2778) (Data not shown).
The differences in the incidences of AEs by demographic factor in the patients with long-term follow-up for 24 weeks or longer showed that there were statistically significant differences; sex (p-value=0.0022), presence or absence of baseline disease (p-value<0.0001), use of concomitant medications (p-value<.0001), the elderly (p-value=0.0003), hepatic impairment (p-value=0.0097), and renal impairment (p-value=0.0004) (Table 4). The differences in the incidences of ADRs regarding the factors showed no statistically significant results; sex (p-value= 0.3725), age (p-value=0.5296), use of concomitant drugs (p-value= 0.3554), and renal impairment (p-value=0.1503). In the factors of presence or absence of baseline disease and patients with hepatic impairment, the results of analysis were consistent with the results of total patients (Data not shown). Overall, the fimasartan was well tolerated, with no deaths reported during the treatment period.
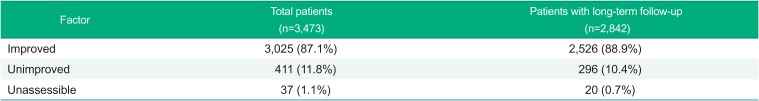
In this study, in order to assess the efficacy after administration of fimasartan the overall improvement rate was classified into ‘Improved’, ‘Unimproved’, or ‘Unassessible’, and consequently the incidence of improved patients was 87.1% (3025 patients), unimproved 11.8% (411 patients), and unassessible 1.1% (37 patients). In the patients with long-term follow-up receiving the fimasartan for 24 weeks or longer, the results of efficacy analysis were similar to the efficacy analysis results of the overall patients (Table 5).
In the clinical symptoms of the total patients for safety assessment performed by visit, the following proportion of the patients showed ‘Improved’ results; 80.6% of patients at visit of ≥0 week to <8 weeks, 79.5% of patients at visit of ≥8 weeks to <16 weeks, 83.2% of patients at visit of ≥16 weeks to <24 weeks, 86.8% of patients at visit of ≥24 weeks to <36 weeks, 88.0% of patients at visit of ≥36 weeks to <48 weeks, and 86.6% of patients at visit of ≥48 weeks. A similar pattern of results was observed in the patients with long-term follow-up for 24 weeks or longer (Table 6).
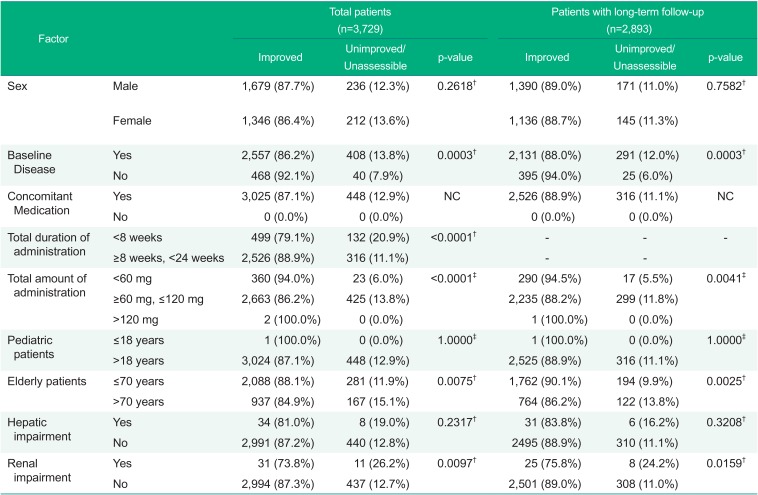
Based on the effects on the efficacy results by each factor, the following showed statistically significant results: presence or absence of baseline disease (p-value=0.0003), total duration of administration of the fimasartan (p-value<0.0001), mean daily dose of fimasartan (p-value<0.0001), the elderly (based on 70 years of age: p-value=0.0075), and renal impairment (p-value=0.0097).
In the patients with long-term follow-up for 24 weeks or longer, statistically significant results were observed in presence or absence of baseline disease (p-value=0.0003), mean daily dose of fimasartan (p=0.0041), the elderly (p=0.0025), and renal impairment (p=0.0159), all of which were consistent with the results in total patients (Table 7).
In addition, the patients with long-term follow-up for 24 weeks or longer had a mean SBP reduction of 14.7±21.3 mmHg from the time of enrollment and a mean DBP reduction of 7.2±13.0 mmHg, which confirmed a statistically significant reduction (Data not shown).
In this post-marketing surveillance that assessed the long-term safety and efficacy of fimasartan in patients with hypertension allowing an opportunity to evaluate the clinical benefit of fimasartan including the long-term safety profile. Among the discontinued patients, in case of poor response, fimasartan were changed to other antihypertensive drug. According to study results in total patients, there were no significant changes in the safety aspect that had not been observed in the clinical study or SAEs and special tendency that had not been reflected at the time of approval. Also, in terms of efficacy, the study showed a significant blood pressure lowering effect after the administration. Such results were also consistently observed in the study results on patients with long-term follow-up for 24 weeks or longer.
The overall incidence of ADRs was 3.8% during this post-marketing surveillance period. According to the J-HEALTH study reported in 2008 with losartan, 1,081 patients out of 29,850 patients (3.6%) had ADRs, which is similar to the results of this study.[14] The incidence of ADRs was 3.1% which is also similar to the result of this study in the large scale Safe-KanArb study that was performed to evaluate the safety and efficacy of fimasartan by the age, sex, concomitant diseases, and ongoing medication of patients with hypertension,[15] although the limited time of evaluation was at week 8 after enrollment. The incidence of AEs showed statistically significance in sex, baseline diseases, concomitant medications, hepatic impairment, and renal impairment in total patients for safety assessment, without any clinically significant pattern. Especially, the incidence of AEs in elderly (based on 70 years of age) had no differences from the results of total patients and the patients with long-term follow-up for 24 weeks or longer, which means there was no difference in safety when used in elderly patients for a long time.
The overall improvement of 3,473 patients for efficacy assessment was analyzed at the last visit. Of 87.1% of the patients were improved, 11.8% were unimproved, and 1.1% were unassessible. The comparison of the improvement rate by demographic factor for 24 weeks or longer showed statistically significant results in baseline diseases, daily dose of fimasartan, the elderly, and renal impairment, however no clinically significance.
This improvement consistently showed in previous study. In the phase III study comparing fimasartan, valsartan, and placebo, the mean SiDBP change in fimasartan at Week 8 was −4.47±8.89 mmHg (vs. valsartan) and the mean SiSBP change in fimasartan was −7.87±15.68 mmHg (vs. valsartan), which there were statistically significant blood pressure lowering effects (p-value= 0.0018, p-value=0.0001).[16]
According to 2017 High Blood Pressure Clinical Practice Guideline revised and recommended by American College of Cardiology (ACC) Foundation and American Heart Association, Inc. (AHA), patients with SBP < 140 mmHg which has not been classified as hypertension are now recommended to modify life style and use prescribed antihypertensive drugs. SBP ≥ 140 mmHg and DBP ≥ 90 mmHg is now classified as stage 2 hypertension which should be managed by strict criteria.[17]
Such criteria developed by applying the results of analysis from approximately 900 related studies including the recent SPRINT study reflected the effect that early management of blood pressure below 130 mmHg reduces the mortality from concomitant diseases by half. Indeed, in accordance with the analysis of data for year 2015 from Korea National Health and Nutrition Examination Survey (KNHANES), 32% of Korean males and females aged 30 years and older (35.1% in males and 29.1% in females) has hypertension as per the previous 140/90 mmHg criterion, however, 50.5% (59.4% in males and 42.2% in females) will have hypertension under the 2017 ACC/AHA criteria. The SPRINT study reported that aggressive blood pressure lowering efforts in American patients aged 50 years and older with hypertension and cardiovascular risk had reduced the primary outcome associated with cardiovascular diseases by 25% and had reduced allcause mortality by 27%.
Fimasartan will be considered to be a more suitable drug than other conventional ARBs, once the updated criteria apply since fimasartan shows a prompt and strong blood pressure lowering effect in this study. SBP was decreased after administration of fimasartan to 131.3±16.2 mmHg at Week 8 from the baseline mean value of 142.3±18.1 mmHg, and further decreased to 130 mmHg after Week 8, and then maintained until the time of long-term prescription. DBP was also decreased to 78.6±11.1 mmHg at Week 8 from the baseline mean value of 83.8±12.8 mmHg and kept at 80 mmHg or lower until the long-term use. A long-term use of fimasartan was capable of controlling blood pressure in a way to satisfy the strict blood pressure control pattern and this study confirmed the safety and efficacy of fimasartan in real-life situation.
This post-marketing surveillance demonstrated that fimasartan has favorable safety and effectiveness profiles for the long-term use in patients with hypertension in Korea.
Acknowledgements
This study was funded by Boryung Pharmaceutical Co., Ltd., Republic of Korea. We also thank to LSK Global Pharma Services Co., Ltd. for data analysis.
References
1. Lawes CM, Vander Hoorn S, Rodgers A. International Society of Hypertension. Global burden of blood-pressure-related disease, 2001. Lancet. 2008; 371:1513–1518. DOI: 10.1016/S0140-6736(08)60655-8. PMID: 18456100.

2. Han MK, Huh Y, Lee SB, Park JH, Lee JJ, Choi EA, et al. Prevalence of stroke and transient ischemic attack in Korean elders: findings from the Korean Longitudinal Study on Health and Aging (KLoSHA). Stroke. 2009; 40:966–969. DOI: 10.1161/STROKEAHA.108.524983. PMID: 19150874.
3. Choi CU, Park CG. Estimating the probability of stroke in Korean hypertensive patients visiting tertiary hospitals using a risk profile from the Framingham study. BMC Neurol. 2009; 9:16. DOI: 10.1186/1471-2377-9-16. PMID: 19386109.

4. Shin CY, Yun KE, Park HS. Blood pressure has a greater impact on cardiovascular mortality than other components of metabolic syndrome in Koreans. Atherosclerosis. 2009; 205:614–619. DOI: 10.1016/j.atherosclerosis.2009.01.014. PMID: 19232617.

5. Murray CJL, Lopez AD. The global burden of disease: a comprehensive assessment of mortality and disability from diseases, injuries, and risk factors in 1990 and projected to 2020. Boston, MA: Harvard University Press;1996. ISBN: 0-9655466-0-8.
6. Antonaccio MJ, Wright JJ. Enzyme inhibitors of the renin-angiotensin system. Prog Drug Res. 1987; 31:161–191. PMID: 3326029.

7. Wood SM, Mann RD, Rawlins MD. Angio-oedema and urticaria associated with angiotensin converting enzyme inhibitors. Br Med J (Clin Res Ed). 1987; 294:91–92.

8. Gravras H, Gravras I. Angiotensin converting enzyme inhibitors. Properties and side effects. Hypertension. 1998; 11:II37–II41.

9. Lee HY, Oh BH. Fimasartan: A new angiotensin receptor blocker. Drugs. 2016; 76:1015–1022. DOI: 10.1007/s40265-016-0592-1. PMID: 27272555.

10. Lee H, Yang HM, Lee HY, Kim JJ, Choi DJ, Seung KB, et al. Efficacy and tolerability of once-daily oral fimasartan 20 to 240 mg/d in Korean Patients with hypertension: findings from Two Phase II, randomized, double-blind, placebo-controlled studies. Clin Ther. 2012; 34:1273–1289. DOI: 10.1016/j.clinthera.2012.04.021. PMID: 22608107.

11. Lee H, Kim KS, Chae SC, Jeong MH, Kim DS, Oh BH. Ambulatory blood pressure response to once-daily fimasartan: an 8-week, multicenter, randomized, double-blind, active-comparator, parallel-group study in Korean patients with mild to moderate essential hypertension. Clin Ther. 2013; 35:1337–1349. DOI: 10.1016/j.clinthera.2013.06.021. PMID: 23932463.

12. Lee SE, Kim YJ, Lee HY, Yang HM, Park CG, Kim JJ, et al. Efficacy and tolerability of fimasartan, a new angiotensin receptor blocker, compared with losartan (50/100 mg): a 12-week, phase III, multicenter, prospective, randomized, double-blind, parallel-group, dose escalation clinical trial with an optional 12-week extension phase in adult Korean patients with mild-to-moderate hypertension. Clin Ther. 2012; 34:552–568. 568.e1–568.e9. DOI: 10.1016/j.clinthera.2012.01.024. PMID: 22381711.

13. Lee JH, Yang DH, Hwang JY, Hur SH, Cha TJ, Kim KS, et al. A randomized, Double-blind, Candesartan-controlled, Parallel group comparison clinical trial to evaluate the antihypertensive efficacy and safety of fimasartan in patients with mild to moderate essential hypertension. Clin Ther. 2016; 38:1485–1497. DOI: 10.1016/j.clinthera.2016.04.005. PMID: 27161546.

14. Naritomi H, Fujita T, Ito S, Ogihara T, Shimada K, Shimamoto K, et al. Efficacy and safety of long-term losartan therapy demonstrated by a prospective observational study in Japanese patients with hypertension: The Japan Hypertension Evaluation with Angiotensin II Antagonist Losartan Therapy (J-HEALTH) study. Hypertens Res. 2008; 31:295–304. DOI: 10.1291/hypres.31.295. PMID: 18360050.

15. Park JB, Sung KC, Kang SM, Cho EJ. Safety and efficacy of fimasartan in patients with arterial hypertension (Safe-KanArb study): an open-label observational study. Am J Cardiovasc Drugs. 2013; 13:47–56. DOI: 10.1007/s40256-013-0004-9. PMID: 23344912.
16. Youn JC, Ihm SH, Bae JH, Park SM, Jeon DW, Jung BC, et al. Efficacy and safety of 30-mg fimasartan for the treatment of patients with mild to moderate hypertension: an 8-week, multicenter, randomized, double-blind, phase III clinical study. Clin Ther. 2014; 36:1412–1421. DOI: 10.1016/j.clinthera.2014.07.004. PMID: 25092393.

17. Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018; 71:1269–1324. DOI: 10.1161/HYP.0000000000000066. PMID: 29133354.

Figure 1
Subject disposition. *Reason for exclusion: (1)=Administered prior to informed consent, (2)=Not administered fimasartan, (3)=Prohibited from receiving fimasarrtan, (4)=Lost to follow-up, (5)=Administration for < 8 weeks, (6)=Efficacy assessment not performed. **Reason for discontinuation: (7)=Poor response, (8)=Adverse events, (9)=No visit, (10)=Others.
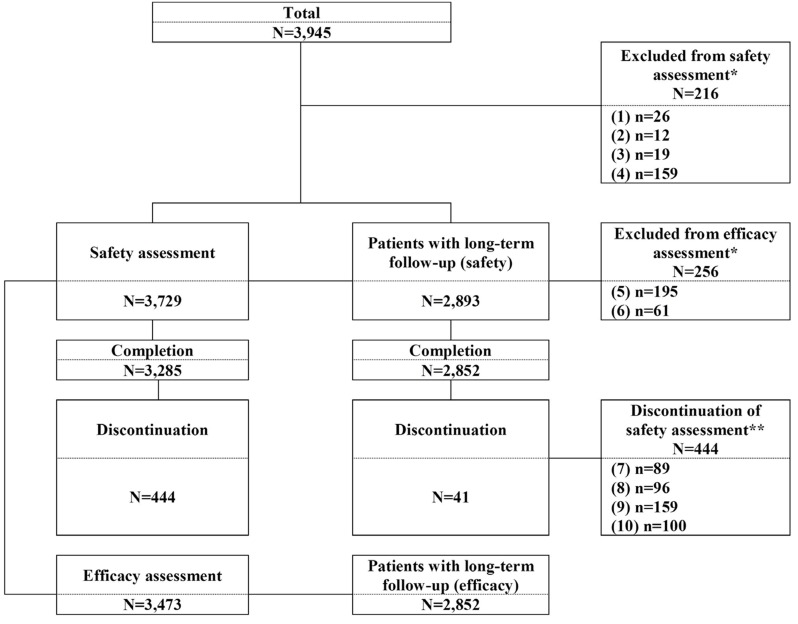
Table 1
Baseline and demographic characterics – Safety assessment
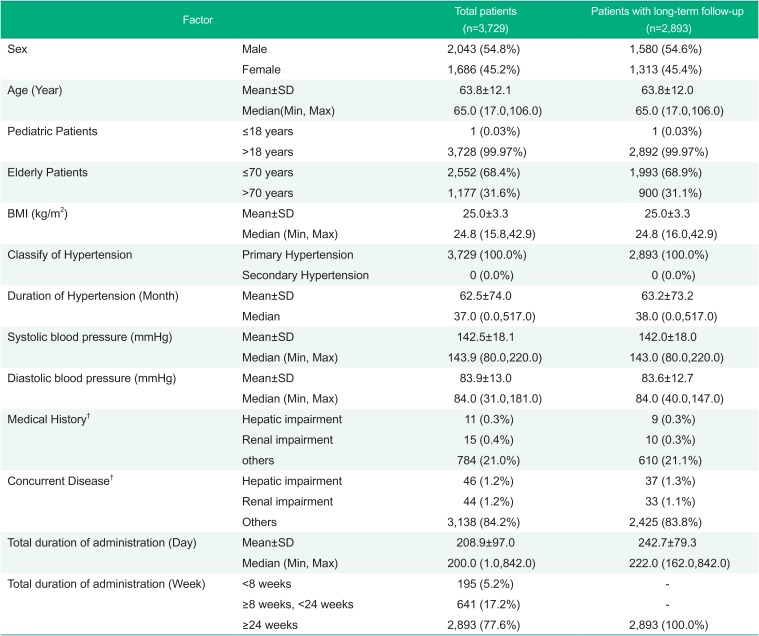
Table 2
Adverse events and Adverse drug reactions – Safety assessment
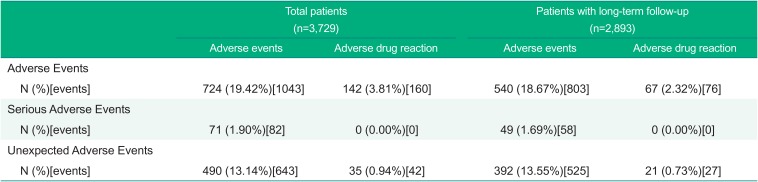
Table 3
Adverse Drug Reactions by System Organ Class and Preferred Term – Safety assessment
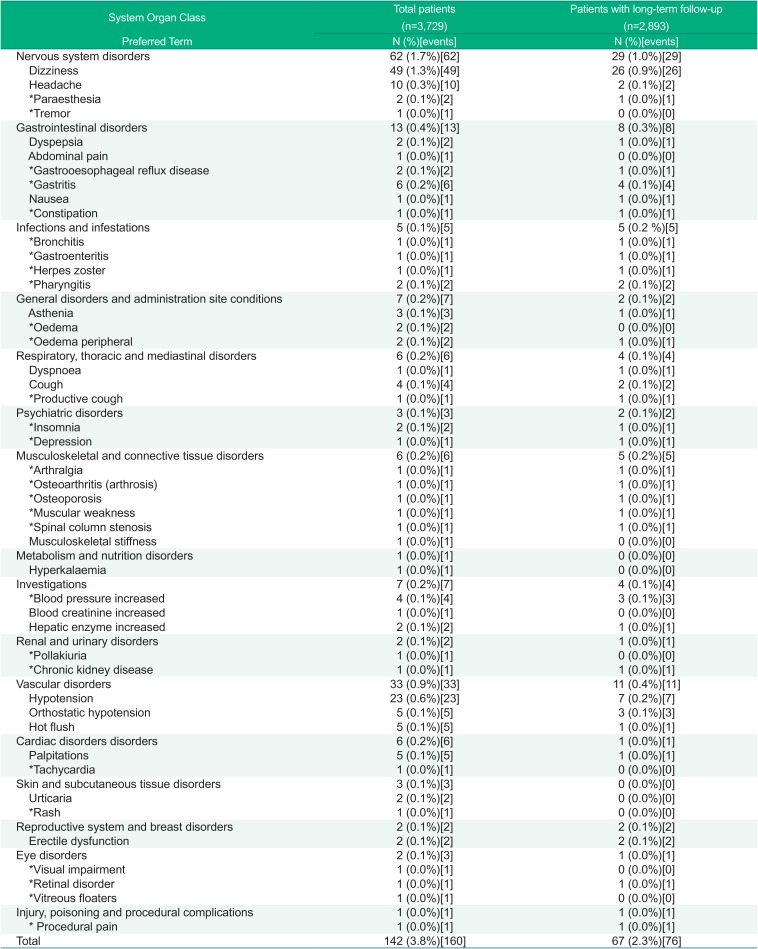
Table 4
The incident of adverse events by factors-Safety assessment
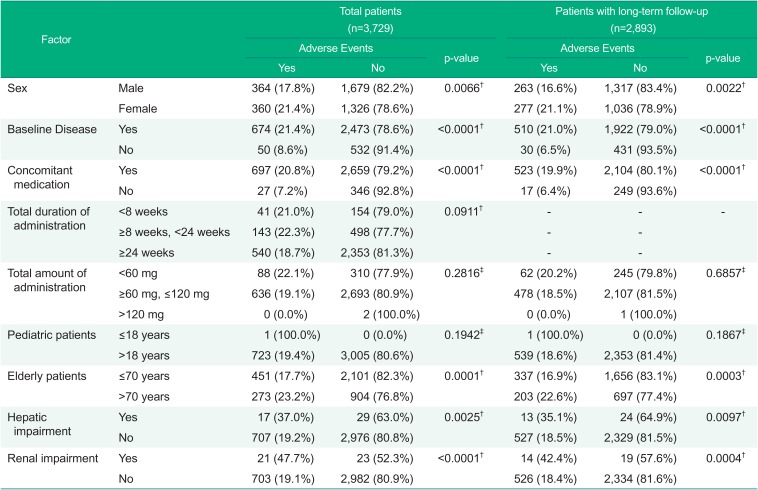
Table 5
The overall improvement-Efficacy assessment
| Factor | Total patients (n=3,473) | Patients with long-term follow-up (n=2,842) |
|---|---|---|
| Improved | 3,025 (87.1%) | 2,526 (88.9%) |
| Unimproved | 411 (11.8%) | 296 (10.4%) |
| Unassessible | 37 (1.1%) | 20 (0.7%) |
Table 6
The clinical symptoms-Efficacy assessment
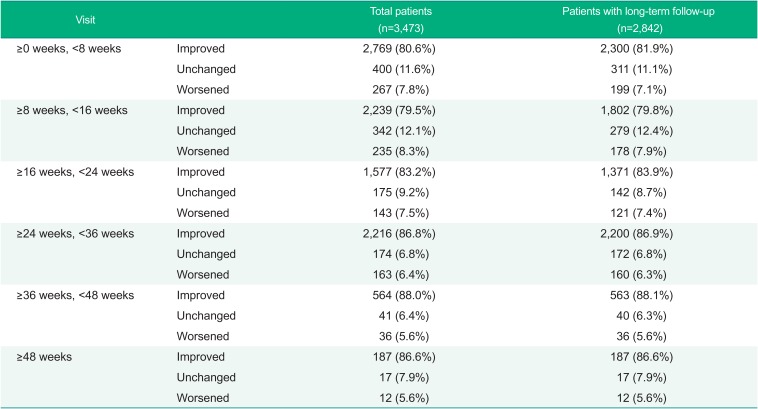
Table 7
The effects on the efficacy results by each factor-Efficacy assessment




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download