Abstract
This study describes the development of an analytical method to determine sumatriptan levels in human plasma using high performance liquid chromatography (HPLC) coupled with triple quadrupole tandem mass spectrometry (MS/MS) and its application to a pharmacokinetic study in healthy Korean volunteers. A single 50 mg dose of sumatriptan was orally administered to twelve healthy volunteers (nine women and three men). The HPLC-MS/MS analytical method was validated with respect to its specificity, linearity, sensitivity, accuracy, precision, recovery, and stability. The calibration curve was linear over a concentration range of 0.3–100 ng/mL (r > 0.999). The lower limit of quantitation for sumatriptan in plasma was 0.3 ng/mL. The accuracy and precision of the analytical method were acceptable within 15% at all quality control levels. We compared plasma concentration-time curves as well as pharmacokinetic parameters such as the area under the curve (AUC) and maximum plasma concentration (Cmax). Both the mean AUC and Cmax of sumatriptan were 1.56 times higher in women than in men. These differences could be largely explained by the difference in body weight (44%) between women and men. The outcomes may provide insights into developing appropriate individualized treatment strategies.
Sumatriptan is a selective 5-hydroxytryptamine (serotonin) type 1B receptor agonist.[12] It is effective for acute migraine treatment and is currently marketed as oral, subcutaneous, intranasal, and suppository (limited distribution) formulations.[3] A pharmacokinetic study showed that sumatriptan was rapidly absorbed and eliminated with a half-life (T1/2) of about 2.4 h after oral administration of a 50-mg dose.[4] The median maximum plasma drug concentration (Cmax) and the time to reach Cmax (Tmax) were ca. 30 ng/mL and 1.3 h, respectively.[4] The area under the curve for 12 h (AUC0–12) was ca. 111 ng·h·mL-1.[4] The bioavailability of oral sumatriptan was low (~14%) due to pre-systemic metabolism and incomplete absorption of the drug after its oral administration.[5]
To develop appropriate individualized treatment strategies, it is important to understand the effects of covariates such as body weight, race, and sex on the pharmacokinetics.[67] Recently Munjal et al. examined the impact of these covariates on the plasma concentration profile of subcutaneous sumatriptan.[8] They showed that exposure was higher in women than in men, which was attributed in part to differences in body weight.[8] The area under the curve during the first 2 hours (AUC0–2), and total area under the curve (AUC0–∞) were lower in non-Caucasians compared with Caucasians.[8] Even though there are many reports on the pharmacokinetics of oral sumatriptan in Caucasians,[891011] the pharmacokinetic study of oral sumatriptan in non-Caucasians is rare. Recently, our group studied the pharmacokinetics of oral sumatriptan in healthy Korean male volunteers using ultrahigh performance liquid chromatography (UPLC) coupled with triple quadrupole tandem mass spectrometry (MS/MS).[1213] However, variability in Korean women's response to oral sumatriptan has not been previously described.
This study describes the development and validation of an analytical method to determine sumatriptan levels in human plasma and its application to a pharmacokinetic study in healthy Korean volunteers. We used high performance liquid chromatography (HPLC) coupled with MS/MS due to the unavailability of an UPLC-MS/MS system. To achieve comparable performance, we modified the previous analytical method and almost fully re-validated it as recommended in the Food and Drug Administration (FDA) guidelines.[1415] This analytical method was applied to a pharmacokinetic study conducted with nine female and three male healthy volunteers.
Sumatriptan succinate (reference standard) was obtained from Tokyo Chemical Industry (Tokyo, Japan). Atenolol (internal standard, IS) was from Sigma-Aldrich (St. Louis, MO, USA). HPLC-grade acetonitrile and ethyl acetate were obtained from Merck (Darmstadt, Germany). ACS reagent-grade formic acid was purchased from Junsei Chemical (Tokyo, Japan). Deionized water (ca. 18 MΩ/cm resistivity) was obtained by using a Milli-Q water purification system (Millipore, Molsheim, France). Sumatriptan succinate for oral administration was obtained from Myung In Pharm (Seoul, Korea). Blank human plasma samples were obtained from healthy Korean volunteers.
An HPLC system (Agilent 1200 series, Santa Clara, CA, USA) was coupled with an MS/MS instrument (API5000; Sciex, Foster City, CA, USA). Chromatographic separation was performed on a Kinetex C18 column (150 × 2.1 mm, 5 µm; Phenomenex, Torrance, CA, USA). Mobile phase, 40% acetonitrile in water with 0.1% formic acid, was filtered through a 0.22 µm membrane filter (Millipore) prior to use. One microliter of sample was injected. An autosampler was maintained at 10℃. The mobile phase was eluted isocratically at a flow rate of 0.2 mL/min, and the column was maintained at 40℃. The eluate was introduced into the mass spectrometer for the targeted analysis of analytes.
The mass spectrometer was operated in a positive electrospray ionization (ESI) mode. Samples were analyzed using a selected reaction-monitoring mode (SRM), which monitors the collision-induced dissociation of a precursor ion to an abundant characteristic product ion. The following transitions of protonated molecular ions, [M+H]+, were selected: m/z 296 → m/z 58 for sumatriptan and m/z 267 → m/z 145 for atenolol. The optimized MS parameters were as follows: source temperature, 600℃; ion spray voltage, 5.5 kV; declustering potential, 111 V; entrance potential, 10 V; curtain gas flow, 12 L/min of nitrogen gas; nebulizing gas flow, 22 L/min of zero air; auxiliary gas flow, 8 L/min of zero air; collision energy, 31 and 37 eV for sumatriptan and atenolol, respectively; collision cell exit potential, 6 and 18 eV for sumatriptan and atenolol, respectively. Analyst 1.5.2 software package (Sciex) was used for instrument control as well as data acquisition and processing.
We prepared primary stock solutions of sumatriptan and atenolol (1 mg/mL) in methanol and water/methanol (50:50, v/v), respectively, and stored at 4℃. We prepared working standard solutions at concentrations of 3, 5, 10, 100, 200, 500, and 1000 ng/mL for sumatriptan and at 1µg/mL for IS by serial dilution of the primary stock solutions. The blank pooled drug-free plasma was spiked with standard solutions to prepare the calibration standards of 0.3, 0.5, 1, 10, 20, 50, and 100 ng/mL of sumatriptan including 500 ng/ml of IS. Quality control (QC) samples were prepared at four concentration levels: 0.3, 0.9, 30, and 80 ng/mL representing the lower limit of quantitation (LLOQ), low, medium, and high QCs, respectively. The calibration standards and QC samples were prepared fresh on the day of analysis.
Plasma samples were stored at –80℃ and allowed to thaw gradually to room temperature before their processing. We transferred an aliquot (200 µL) of the plasma sample to a clean tube and added 20 µL of the IS solution (0.5 µg/mL). After the tube was briefly vortexed, 1.2 mL of ethyl acetate was added. This mixture was vortexed for 5 min and then centrifuged at 13,200 rpm for 5 min at 4℃. The supernatant was transferred to a clean tube and evaporated to dryness in a SpeedVac vacuum evaporator (Savant Instruments, Holbrook, NY, USA) for approximately 30 min at 45℃. The dry residue was reconstituted in 300 µL of the eluent, and vortexed for 3 min, and centrifuged at 13,200 rpm for 5 min at 4℃. The supernatant (100 µL) was then transferred to an HPLC vial for further HPLC-MS/MS analysis.
We evaluated the specificity of the method by analyzing drug-free plasma samples from six different individuals. We examined any interference at the LC retention times of sumatriptan and IS by comparing the chromatograms of a double blank, plasma sample without sumatriptan and IS, with those of blank plasma samples spiked with sumatriptan at LLOQ (0.3 ng/mL) and/or IS (500 ng/mL).
Calibration curves were obtained by plotting the peak area ratio of sumatriptan to IS vs. the nominal sumatriptan in the concentration range of 0.3–100 ng/mL. A weighted (1/χ) least-squares regression was used to evaluate its linearity. Sensitivity is represented by the LLOQ, which is considered being the lowest calibration standard. The analyte signal of the LLOQ sample should be at least ten times as large as the signal of a blank sample when carry-over effects are considered.
The intra-day accuracy and precision were assessed by analyzing five replicates of each QC sample (0.3, 0.9, 30, and 80 ng/mL, respectively) on the same day. The inter-day accuracy and precision were determined by analyzing the QC samples on five different days. The accuracy was calculated as (measured concentration/nominal concentration) × 100%, and the precision was expressed as the relative standard deviation (RSD). Acceptable accuracy and precision for all concentration levels were set within ±15% deviation, except at the LLOQ concentration level (within ±20%).
Sumatriptan recovery was determined in triplicates at three concentrations (0.9, 30, and 80 ng/mL). Absolute recoveries were determined by comparing the peak area ratio of the spiked sumatriptan in a blank plasma sample before and after extraction at the corresponding concentrations. IS recovery was determined at a single concentration (500 ng/mL) level in a similar manner.
Stability was measured at the low (0.9 ng/mL) and high (80 ng/mL) QC concentrations in triplicate. We tested four stability conditions: post preparation stability at 10℃ for 24 h, freeze/thaw stability for three cycles, short-term stability at room temperature for 6 h, and long-term stability at –80℃ for 211 days. The concentrations obtained from all stability studies were analyzed with the same calibration curve as described above and compared to those of freshly prepared QC samples.[1415]
We applied the validation method to a pharmacokinetic study of sumatriptan among healthy volunteers. The demographic information of study subjects is summarized in Table 1. The institutional review board of Kyungpook National University Hospital (Daegu, Korea) approved the pharmacokinetic study protocol. All the volunteers provided written informed consent. After an overnight fast of 10 h, the volunteers received a single, 50-mg oral dose of sumatriptan with 150 mL of water. Additional water intake was permitted 2 h after dosing. No volunteers were allowed to take any concomitant medication during the study period. Blood samples (8 mL) were collected in tubes containing sodium heparin before (0 h) and at 0.25, 0.5, 0.75, 1, 1.5, 2, 2.5, 3, 4, 6, 8, 10, and 12 h after sumatriptan administration. The plasma samples were immediately separated from the blood via centrifugation at 3000 rpm (800×g) for 10 min at 4℃ and were stored at –80℃ until subsequent analysis. We used Phoenix WinNonlin® 6.4 (Certara, NJ, USA) to calculate the pharmacokinetic parameters of sumatriptan including AUC, Cmax, Tmax, and T1/2.
To determine characteristic transitions for the selected reaction monitoring of sumatriptan and IS, we performed MS/MS experiments in positive and negative ESI modes. We directly infused each solution in the mobile phase (1 µg/mL) into the mass spectrometer at a flow rate of 0.2 mL/min. MS/MS parameters were tuned to increase sensitivity for respective product ions. Positive ion mode showed significantly greater ion abundances than did negative ion mode. Sumatriptan and IS yielded protonated molecular ions ([M+H]+) at m/z 296 and 267, respectively, in positive ion mode. The fully scanned mass spectra presented the most abundant and stable product ions at m/z 58 and 145 for sumatriptan and IS, respectively.
Analysis of sumatriptan was initiated under isocratic conditions as before.[13] Previously, the mobile phase of water–acetonitrile–formic acid at 83:17:0.1 (v/v/v) was used for the chromatographic separation of sumatriptan and terazosin.[13] For better performance, we adjusted the polarity of the mobile phase and decided to use water–acetonitrile–formic acid at 60:40:0.1 (v/v/v) as a mobile phase in this study. In addition, we chose atenolol as an IS instead of terazosin because of the similar chemical property of sumatriptan and atenolol. Sumatriptan and IS were eluted from the column at 1.50 and 1.49 min, respectively, with good peak symmetry and reproducibility (data not shown here).
Previously, the mean percent recovery rates of sumatriptan were 69.8, 72.9, and 62.3% at concentrations of 1, 10, and 50 ng/mL, respectively, and the RSDs (%) of these values were within 11.8%.[13] In this study, the mean percent recovery rates of sumatriptan were 77.7, 77.2, and 67.7% for the plasma samples (n = 3) at concentrations of 0.9, 30, and 80 ng/mL, respectively. Furthermore, the RSDs of these values were within 3.4%. The improvement in the mean percent recovery rate and RSD can be attributed to the higher similarity of sumatriptan to atenolol in chemical properties than to terazosin.
In this study, we investigated the specificity using independent drug-free plasma. We compared the SRM chromatograms of the following four samples: blank human plasma, blank human plasma spiked with sumatriptan at the LLOQ (0.3 ng/mL), blank human plasma spiked with IS (500 ng/mL), and blank human plasma spiked with both sumatriptan and IS. The chromatograms did not show any interfering endogenous peaks at the measured mass transitions and retention times of sumatriptan and IS in the highly selective SRM mode.
The calibration curves were linear over the concentration range of 0.3–100 ng/mL with a correlation coefficient (r) of 0.999 or better. The measured LLOQ was 0.3 ng/ml. The sensitivity of this method was slightly higher than that of the previous method (0.5 ng/mL),[13] and sufficient for the determination and pharmacokinetic analysis of sumatriptan in human plasma.
Table 2 presents the intra- and inter-day precision and accuracy at four QC levels (0.3, 0.9, 30, and 80 ng/mL). The RSDs for all QC samples were within 11.2%, while the relative errors (REs) in the accuracy were less than 6.3%. Thus, all QC samples met the currently accepted criteria of ±20% precision and accuracy at the LLOQ concentration level and ±15% for higher concentrations.
Table 3 summarizes the results from stability tests. The QC plasma samples at two concentrations (0.9 and 80 ng/mL) were stable during sample preparation procedures, storage, and after sample extraction. Stored sumatriptan and IS stock solutions were stable for up to 211 days at −80℃. Sumatriptan levels in human plasma varied only slightly as shown in Table 3. This indicates adequate sample stability under the examined conditions.
We applied the validated analytical method to a pharmacokinetic study of sumatriptan after oral administration of a 50-mg dose to nine female and three male healthy volunteers. The sumatriptan concentrations in plasma were higher than LLOQ and quantifiable at all the time points. Figure 1 shows the mean concentration–time profiles of sumatriptan in human plasma for women and men. In general, the exposure levels of women to oral sumatriptan were at least 40% or higher than those of men at each time point. Due to relatively high standard deviation at each time point, however, we cannot confirm if there are clear differences in the mean concentration-time profiles of sumatriptan between the two groups.
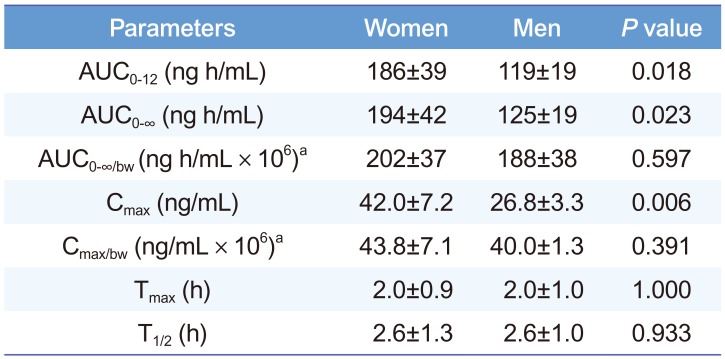
Table 4 presents calculated pharmacokinetic parameters. T1/2 and Tmax were similar between both groups. However, both Cmax and AUC0-∞ of sumatriptan were 1.56 times higher in women than in men. In this study, men were 44% heavier than women were (Table 1). Therefore, relatively higher exposures in women are natural because of their generally lower body weight. To compensate for the differences in body weight, Cmax and AUC0-∞ were divided by body-weight normalized dosage to give Cmax/bw and AUC0-∞/bw, respectively. The body-weight adjusted parameters were still 7–9% higher in women than in men (Table 4). However, Student's t-test results show that the differences in Cmax and AUC0-∞ were not significant (P > 0.05) after correcting for body-weight differences.
Previously, we demonstrated the successful development of an analytical method to determine sumatriptan levels in human plasma using UPLC-MS/MS.[13] Due to the unavailability of an UPLC-MS/MS system, however, we used an HPLC-MS/MS system in this study. To achieve comparable chromatographic performance, we replaced terazosin with atenolol as an IS. Furthermore, we increased the acetonitrile content in the mobile phase to shorten overall retention times. Thus, the chromatographic analysis of sumatriptan and IS in human plasma could be performed within 5 min as before. The validation results in the current study were also comparable to those obtained in the previous study.[13] The method accuracies were within 15%, and the RSDs of the intra- and inter-day precision were also within 15% at all quality control levels. The LLOQ was sufficiently low to quantitate sumatriptan concentrations at all the time points of interest. Additionally, the pharmacokinetic parameters of sumatriptan for healthy male volunteers in this study, including AUC0-∞ and Cmax, were comparable not only to those previously reported for healthy male volunteers [1213] but also to those obtained by Moore et al.[4] However, these parameters for men were significantly different from those for women with a ratio of 1.56. Munjal et al. studied the statistically significant impact of body size [weight and body mass index (BMI)] and sex on systemic exposure to subcutaneously administered sumatriptan.[8] They showed that sumatriptan exposure in women was higher than that in men with a typical ratio of ~1.2. Both body weight and BMI were important covariates for sumatriptan exposure. In this study, however, it is unlikely that BMI (17% difference between the two groups) is a major factor to justify the observed difference in pharmacokinetic parameters. When data were adjusted for body weight, the differences in pharmacokinetic parameters became non-significant. This indicates that the lower body weight of women significantly correlates with the higher exposure of women than that of men. Nevertheless, care must be taken in interpreting our data to determine whether doses of orally administered sumatriptan should be adjusted based on body weight for comparable efficacy, because the sample size was small in this study. Detailed studies with a larger sample size will be required to understand better the mechanisms and observed sex-specific differences in pharmacokinetics.
In conclusion, we developed and validated an HPLC-MS/MS analysis method for the quantitation of sumatriptan in human plasma. We successfully applied this method to a clinical pharmacokinetic study of sumatriptan in healthy Korean female and male volunteers. By comparing the pharmacokinetic parameters of sumatriptan between women and men, we showed that the difference in body weight between women and men had a significant effect on sumatriptan pharmacokinetics. However, sex difference was not significant after the pharmacokinetic parameters were adjusted for body weight. Despite our study being limited by a small sample size, our preliminary results will be useful to develop a larger scale studies and to advance our understanding of sex-related difference in the pharmacokinetics of sumatriptan.
Acknowledgements
This study was supported by a grant of the Korean Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (HI14C2750). This work was supported by the Industrial Core Technology Development Program (10051129, Development of the system for ADME assessment using radiolabeled compounds) funded by the Ministry of Trade, Industry & Energy (MOTIE, Korea).
References
1. Humphrey PP. The discovery of a new drug class for the acute treatment of migraine. Headache. 2007; 47(Suppl 1):S10–S19. PMID: 17425704.

2. Hoyer D, Clarke DE, Fozard JR, Hartig PR, Martin GR, Mylecharane EJ, et al. International Union of Pharmacology classification of receptors for 5-hydroxytryptamine (Serotonin). Pharmacol Rev. 1994; 46:157–203. PMID: 7938165.
3. Duquesnoy C, Mamet JP, Sumner D, Fuseau E. Comparative clinical pharmacokinetics of single doses of sumatriptan following subcutaneous, oral, rectal and intranasal administration. Eur J Pharm Sci. 1998; 6:99–104. PMID: 9795022.

4. Moore KH, Leese PT, McNeal S, Gray P, O'Quinn S, Bye C, et al. The phar-macokinetics of sumatriptan when administered with clarithromycin in healthy volunteers. Clin Ther. 2002; 24:583–594. PMID: 12017403.

5. Lacey LF, Hussey EK, Fowler PA. Single dose pharmacokinetics of sumatriptan in healthy volunteers. Eur J Clin Pharmacol. 1995; 47:543–548. PMID: 7768259.

7. Federman DD. The biology of human sex differences. N Engl J Med. 2006; 354:1507–1514. PMID: 16598047.

8. Munjal S, Gautam A, Rapoport AM, Fisher DM. The effect of weight, body mass index, age, sex, and race on plasma concentrations of subcutaneous sumatriptan: a pooled analysis. Clin Pharmacol. 2016; 8:109–116. DOI: 10.2147/CPAA.S108966. PMID: 27621672.
9. Sternieri E, Pinetti D, Coccia CP, Leone S, Bertolini A, Ferrari A. Pharmacokinetics of sumatriptan in non-respondent and in adverse drug reaction reporting migraine patients. J Headache Pain. 2005; 6:319–321. PMID: 16362699.

10. Ferrari A, Pinetti D, Bertolini A, Coccia C, Sternieri E. Interindividual variability of oral sumatriptan pharmacokinetics and of clinical response in migraine patients. Eur J Clin Pharmacol. 2008; 64:489–495. DOI: 10.1007/s00228-007-0443-9. PMID: 18180912.

11. Rani PU, Naidu MUR, Kumar TR, Shobha JC, Vijay T, Sekhar KR, et al. A bioequivalence study of two brands of sumatriptan tablets. Curr Ther Res Clin E. 1996; 57:589–598.
12. Lee J, Lim MS, Seong SJ, Park SM, Gwon MR, Han S, et al. Population pharmacokinetic analysis of the multiple peaks phenomenon in sumatriptan. Transl Clin Pharmacol. 2015; 23:66–74.

13. Seo JJ, Park J, Bae MH, Lim MS, Seong SJ, Lee J, et al. Rapid determination of sumatriptan in human plasma by ultra performance liquid chromatography-tandem mass spectrometry and its application to clinical pharmacokinetic study. J Chromatogr B Analyt Technol Biomed Life Sci. 2013; 919-920:38–42. DOI: 10.1016/j.jchromb.2013.01.004.

14. US Food and Drug Administration. Guidance for Industry: Bioanalytical method validation. Accessed 11 May 2017. http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm070107.pdf.
15. Korea Food and Drug Administration. Guideline on bioanalytical method validation. Accessed 11 May 2017. http://www.mfds.go.kr/index.do?mid=1161&seq=7560.
Figure 1
Mean plasma concentration-time profiles of sumatriptan after single oral dose 50-mg sumatriptan for nine female (blue open circle) and three male (red closed circle) healthy volunteers. Shaded areas with error bars represent standard deviation from the mean value.
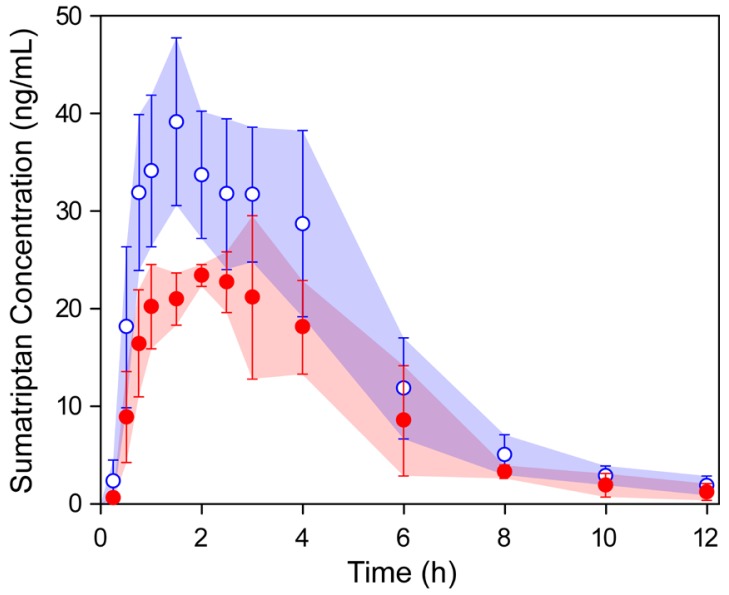
Table 1
Demographic characteristics of study subjects
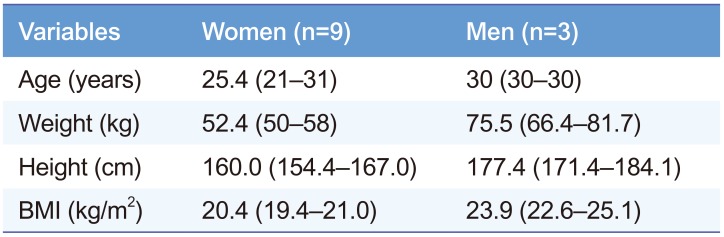
Table 2
Intra- and inter-day (n=5) accuracy and precision of QC samples of sumatriptan in plasma.
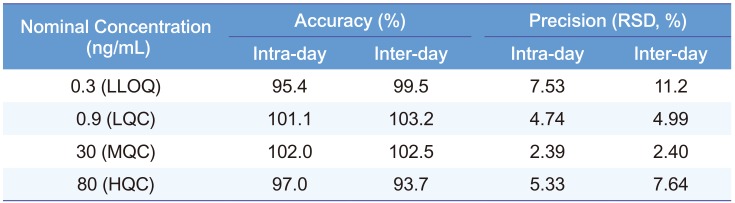
Table 3
Stability of sumatriptan standard in plasma under four different conditions (n=3)
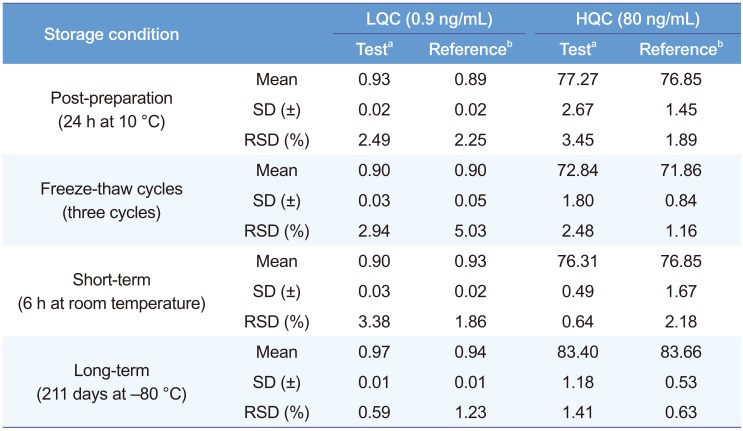
Table 4
Calculated pharmacokinetic parameters of sumatriptan after oral administration of 50-mg to healthy nine female and three male volunteers




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download