Abstract
This study evaluates the suitability of cadavers embalmed by the ethanol-glycerin fixative for the dissection course of medical students and the hands-on dissection workshop of clinicians. Five cadavers were embalmed by two different methods: two formalin-phenol fixation (FPF) and three ethanol-glycerin fixation (EGF) cadavers. The measurement of physical and chemical characteristics including ranges of motion (ROM), bacterial and fungal culture tests, and ultrasonography were performed for each cadaver.
The EGF cadavers were evaluated to be significantly more suitable than FPF cadavers for the physical and chemical characteristics including color, texture, elasticity, wetness (softness), skin incision, vessel ligation and suture, decollement, odor, and irritant. In shoulder, elbow, and wrist joints, ROMs of the EGF cadavers were statistically more than those of the FPF except for elbow extension. On bacterial and fungal culture tests at 8 weeks after carrying out of refrigerator, one bacteria were detected in one EGF cadaver; however, some bacteria and fungi could be detected in all FPF cadavers. The ultrasound images of abdominal organ and thigh musculature could be more clearly detected in the EGF cadavers than those of FPF cadavers.
These results indicate that the EGF method had a sufficient antibiotic effect and produced cadavers with flexible joints and a high tissue quality suitable for various cadaveric dissection courses.
References
1. Drake RL, McBride JM, Lachman N, Pawlina W. Medical education in the anatomical sciences: the winds of change continue to blow. Anat Sci Educ. 2009; 2:253–9.

2. Pawlina W. Basic sciences in medical education: Why? How? When? Where? Med Teach. 2009; 31:787–9.

3. Orbson CP, Kaiser RS, Ross CF. Physician opinions about an anatomy core curriculum: a case for medical imaging and vertical integration. Anat Sci Educ. 2013; 7:251–61.
4. Sugand K, Abrahams P, Khurana A. The anatomy of anatomy: a review for its modernization. Anat Sci Educ. 2010; 3:83–93.

5. Wittich CM, Pawlina W, Drake RL, Szostek JH, Reed DA, Lachman N, et al. Validation of a method for measuring medical students'critical reflections on professionalism in gross anatomy. Anat Sci Educ. 2013; 6:232–8.
6. Hayashi S, Homma H, Naito M, Oda J, Nishiyama T, Kawamoto A, et al. Saturated salt solution method: a useful cadaver embalming for surgical skills training. Medicine. 2014; 93:e196.
7. Macchi V, Munari PF, Brizzi E, Parenti A, De Caro R. Workshop in clinical anatomy for residents in gynecology and obstetrics. Clin Anat. 2003; 16:440–7.

8. Yang JH, Kim YM, Chung HS, Cho J, Lee HM, Kang GH, et al. Comparison of four manikins and fresh frozen cadaver models for direct laryngoscopic orotracheal intubation training. Emerg Med. 2010; J27:13–6.

9. Lewis CE, Peacock WJ, Tillou A, Hines OJ, Hiatt JR. A novel cadaver-based educational program in general surgery training. J Surg Educ. 2012; 69:693–8.

10. Sharma M, Macafee D, Horgan AF. Basic laparoscopic skills training using fresh frozen cadaver: a randomized controlled trial. Am J Surg. 2013; 206:23–31.

11. Anderson SD. Practical light embalming technique for use in the surgical fresh tissue dissection laboratory. Clin Anat. 2006; 19:8–11.

12. Balta JY, Cronin M, Cryan JF, O'Mahony SM. Human preservation techniques in anatomy: A 21st century medical education perspective. Clin Anat. 2015; 28:725–34.

13. Coleman R, Kogan I. An improved low-formaldehyde embalming fluid to preserve cadavers for anatomy teaching. J Anat. 1998; 192:443–6.

14. Brenner E. Human body preservation-old and new techniques. J Anat. 2014; 224:316–44.
15. Richins CA, Roberts EC, Zeilmann JA. Improved fluids for anatomical embalming and storage. Anat Rec. 1963; 146:241–3.

16. Wilke HJ, Werner K, Haussler K, Reinehr M, Böckers TM. Thiel-fixation preserves the nonlinear load-deformation characteristic of spinal motion segments, but increases their flexibility. J Mech Behav Biomed Mater. 2011; 4:2133–7.

17. Hauptmann M, Stewart PA, Lubin JH, Beane Freeman LE, Hornung RW, Herrick RF, et al. Mortality from lymphohematopoietic malignancies and brain cancer among embalmers exposed to formaldehyde. J Natl Cancer Inst. 2009; 101:1696–708.

18. National Toxicology Program. Final report on carcinogens background document for formaldehyde. Rep Carcinog Backgr Doc. 2010; 10–2981:i–512.
19. Hammer N, Löffler S, Feja C, Sandrock M, Schmidt W, Bechmann I, et al. Ethanol-glycerin fixation with thymol conservation: A potential alternative to formaldehyde and phenol embalming. Anat Sci Educ. 2012; 5:225–33.

20. Spranger H. Die Verwendung von Isopropylalkohol statt Äthylalkohol in der pathologisch-anatomischen Technik. Zbl Path Jena. 1926; 38:65–7.
21. Jaung R, Cook P, Blyth P. A comparison of embalming fluids for use in surgical workshops. Clin Anat. 2011; 24:155–61.

22. Wineski LE, English AW. Phenoxyethanol as a nontoxic preservative in the dissection laboratory. Acta Anat (Basel). 1989; 136:155–8.

23. Whitehead MC, Savoia MC. Evaluation of methods to reduce formaldehyde levels of cadavers in the dissection laboratory. Clin Anat. 2008; 21:75–81.

24. Messmer C, Kellogg RT, Zhang Y, Baiak A, Leiweke C, Marcus JR, et al. A technique to perfuse cadavers that extends the useful life of fresh tissues: The Duke experience. Anat Sci Educ. 2010; 3:191–4.

25. Schramek GG, Stoevesandt D, Reising A, Kielstein JT, Hiss M, Kielstein H. Imaging in anatomy: a comparison of imaging techniques in embalmed human cadavers. BMC Med Educ. 2013; 13:143.

26. Wolff KD, Kesting M, Mücke T, Rau A, Hölzle F. Thiel embalming technique: a valuable method for microvascular exercise and teaching of flap raising. Microsurgery. 2008; 28:273–8.

27. Benkhadra M, Faust A, Ladoire S, Trost O, Trouilloud P, Girard C, et al. Comparison of fresh and Thiel's embalmed cadavers according to the suitability for ultrasound-guided regional anesthesia of the cervical region. Surg Radiol Anat. 2009; 31:531–5.

28. Luyet C, Eichenberger U, Greif R, Vogt A, Szücs Farkas Z, Moriggl B. Ultrasound-guided paravertebral puncture and placement of catheters in human cadavers: an imaging study. Br J Anaesth. 2009; 102:534–9.

29. Fessel G, Frey K, Schweizer A, Calcagni M, Ullrich O, Snedeker JG. Suitability of Thiel embalmed tendons for biomechanical investigation. Ann Anat. 2011; 193:237–41.

30. Bisht D, Pal A, Chanotiya CS, Mishra D, Pandey KN. Terpenoid composition and antifungal activity of three commercially important essential oils against Aspergillus flavus and Aspergillus niger. Nat Prod Res. 2011; 25:1993–8.
Fig. 1.
Time frame of study design. FPF: formalin-phenol fixation, EGF: ethanol-glycerin fixation, HSEA: high-speed embalming apparatus, Fridge: carrying cadavers out of the refrigerator, P&T: All procedures and tests, 2°CT: second culture test, wk: week.
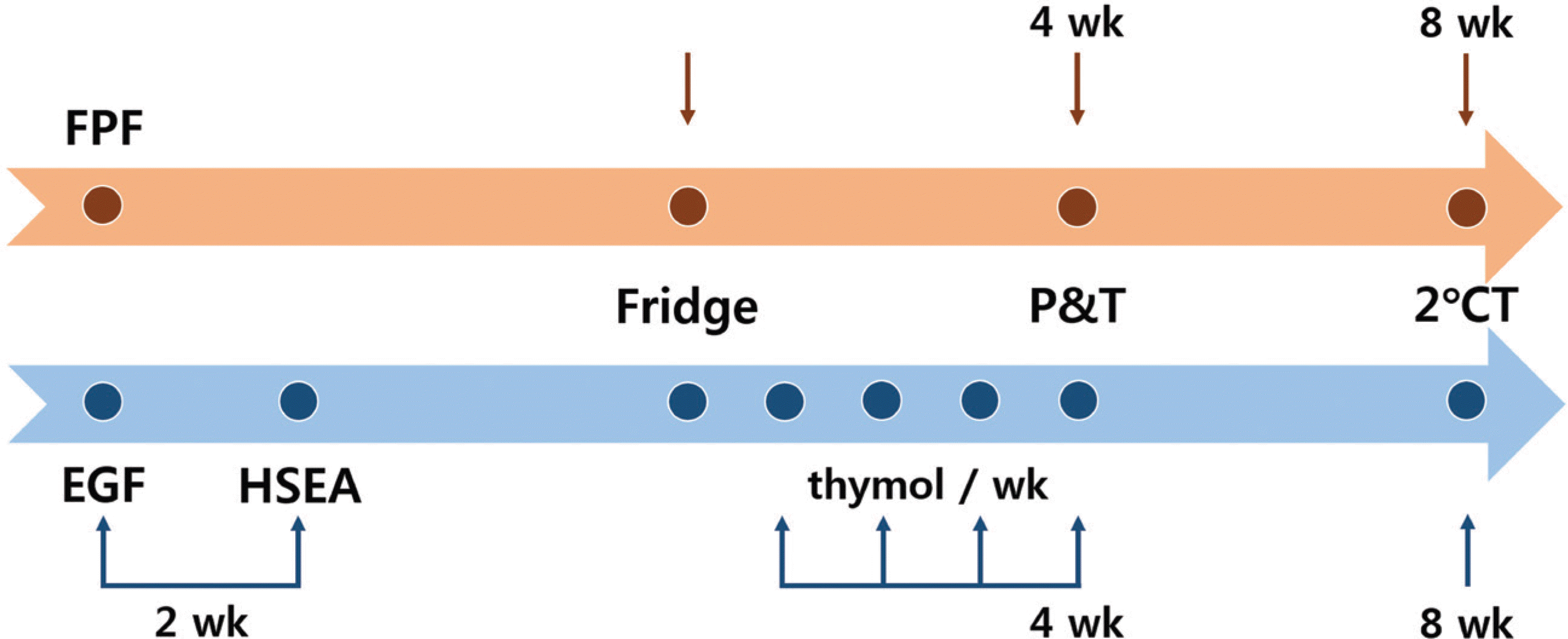
Fig. 2.
Assessment of physical and chemical characteristics in cadavers embalmed by two different methods. (A) to (B) are formalin-phenol solution-fixed cadavers, whereas (C) to (F) are ethanol-glycerin solution-fixed cadavers. (A) and (D): skin incision and decollement; (B): vessel ligation; (E): small intestine; (C) and (F): the vasculature of meninges and brain.

Fig. 3.
Assessment of the range of motion in cadavers embalmed by two different methods. (A) to (C) are formalin-phenol solution-fixed cadavers, whereas (D) to (F) are ethanol-glycerin solution-fixed cadavers. (A) and (D): shoulder abduction; (B) and (E): elbow flexion; (C) and (F): wrist flexion.

Fig. 4.
Ultrasonography in cadavers embalmed by two different methods. (A) to (C) are from the formalin-phenol solution-fixed cadavers, whereas (D) to (F) are from ethanol-glycerin solution-fixed cadavers. (A) and (D) images through right upper quadrant in abdomen; (B) and (E) images through pelvis; (C) and (F) images through right thigh.
Table 1.
General characteristics of donated cadavers
Table 2.
The protocol of formalin-phenol fixation summarizes the amounts and concentrations of fixatives, duration of each step, and required conditions
Table 3.
The protocol of ethanol-glycerin fixation and thymol conservation summarizes the amounts and concentrations of fixatives, duration of each step, and required conditions
Table 4.
Comparison of the physical and chemical characteristics in cadavers following embalming by two different methods
Table 5.
Range of motion (ROM) of joints in cadavers following embalming by two different methods
Table 6.
Results of bacterial and fungal culture tests on cadaver following two different embalming methods




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download