Abstract
Purpose
Prostate calcifications are a common finding during transrectal prostate ultrasound in both healthy subjects and patients, but their etiopathogenesis and clinical significance are not fully understood. We aimed to establish a new methodology for evaluating the role of microbial biofilms in the genesis of prostate calcifications.
Materials and Methods
Ten consecutive patients who had undergone radical prostatectomy were enrolled in this study. All of the patients presented with prostate calcifications during transrectal ultrasound evaluation before surgery and underwent Meares-Stamey tests and clinical evaluation with the National Institutes of Health Chronic Prostatitis Symptom Index and the International Prostate Symptom Score. At the time of radical prostatectomy, the prostate specimen, after removal, was analyzed with ultrasonography under sterile conditions in the operating room. Core biopsy specimens were taken from the site of prostate calcification and subjected to ultrastructural and microbiological analysis.
Results
The results of the Meares-Stamey test showed only 1 of 10 patients (10%) with positive cultures for Escherichia coli. Two of five patients (40%) had positive cultures from prostate biopsy specimens. Enterococcus faecalis, Enterococcus raffinosus, and Citrobacter freundii were isolated. Ultrastructural analysis of the prostate biopsy specimens showed prostate calcifications in 6 of 10 patients (60%), and a structured microbial biofilm in 1 patient who had positive cultures for E. faecalis and E. raffinosus.
Prostate calcifications are a common finding during transrectal prostate ultrasound in both healthy subjects and patients undergoing prostate biopsy [1,2], but their clinical role and etiopathogenesis is not fully understood. Some authors state that prostate stones and calcifications are the result of inspissated prostatic secretions, with a core surrounded by concentric layers of calcium apatite [3]. Prostate calcifications may also be the result of an inflammatory process because of aging or intraprostatic reflux. Calcifications have been reported to progress and cause mechanical obstruction, smooth muscle contraction, and voiding symptoms [4,5]. However, we are still far from understanding the clinical significance and etiopathogenesis of prostate calcifications. It is generally accepted that the incidence of prostatic calcification increases with age [6] and that the incidence is somehow related to chronic prostatitis or chronic pelvic pain syndrome in young men [5,7]. Moreover, prostate calcifications seem to play an important role in lower urinary tract symptoms (LUTS), but the relationship between prostate calcifications and LUTS remains elusive [1].
Recently, interest in the role of biofilm-producing bacteria in the development of acute and chronic prostatitis has increased [8,9]. Bartoletti and coworkers demonstrated the role of biofilm-producing bacteria for the persistence of symptoms in patients with chronic bacterial prostatitis, irrespective of the administration of antibiotic treatment [9]. From these observations, we hypothesized that biofilm-producing bacteria may play a role in the genesis of prostate calcifications. In the present study, we tested a new methodology for identifying microbial biofilms at the calcification surface by using microbial culture and ultrastructural characterization techniques in a small cohort of patients.
Ten consecutive patients who underwent radical prostatectomy for prostate cancer were enrolled in this study between January and June 2015. Subjects were selected from patients who had prostate calcifications as demonstrated on transrectal ultrasound-guided prostate biopsy. The day before surgery, all patients underwent a clinical evaluation, underwent Meares-Stamey testing, and filled in the National Institutes of Health Chronic Prostatitis Symptom Index (NIH-CPSI) and the International Prostate Symptom Score (IPSS) questionnaire. Immediately after removal, the prostates were examined by ultrasonography under sterile conditions in the operating room. One or two core biopsy specimens were taken from the site of prostate calcification and subjected to microbiological and ultrastructural analyses.
All samples from the Meares-Stamey test were collected and immediately brought to the laboratory under refrigeration. Aliquots were analyzed for cultures and processed for DNA extraction and polymerase chain reaction as described by Mazzoli et al. [12] and Cai et al. [13]. All specimens were examined for microbiological cultures of common bacteria and yeasts, and DNA extraction and mucosal immunoglobulin A evaluation was performed for diagnosis of Chlamydia trachomatis infection. All patients underwent the Meares-Stamey test as described by Mazzoli et al. [12]. Microorganisms were identified and tested for antibiotic susceptibility by using the Vitek II semiautomated system for microbiology (BioMerieux, Florence, Italy) [12,13].
From the first five patients, two prostate core biopsy specimens were obtained for both ultrastructural and microbiological analyses, respectively. From the subsequent five patients, only one prostate core biopsy specimen was available for ultrastructural analysis from each patient. Each biopsy specimen was obtained under sterile conditions from the removed prostate, immediately after surgery, and collected in a screw-capped sterile container with 5 mL of sterile saline. All collected biopsy specimens were sent to the laboratory for ultrastructural and microbiological analyses within 2 hours of sampling.
Each biopsy specimen was fixed in phosphate-buffered 4% formalin for ultrastructural analysis by scanning electron microscopy (SEM). After 24 hours of fixation, the specimen was washed twice in distilled water, dehydrated in ascending hydro-alcoholic solutions, vacuum dried, and mounted on an aluminum stub with carbon-conductive and gold-sputtered tape. The sample was imaged in an XL30 FEG-FEI scanning electron microscope in high-vacuum mode at a magnification ranging from 50× to 10,000×. Any micromorphology compatible with microbial biofilm or tissue calcification was inspected and documented by collecting a set of digital micrographs, in line with Cai et al. [14]. The crystal composition of calcifications was assessed by using the energy-dispersive X-ray spectroscope integrated in the SEM, in line with Cai et al. [15].
Prostate biopsy specimens were homogenized in 5 mL of sterile saline by using a tissue grinder (Ultra Turrax; IKA-Werke, Staufen, Germany) to dislodge the bacterial biofilm from tissues and calcifications. Homogenate fluid was then concentrated by centrifugation at 3,500 rpm for 10 minutes, and 10 µL of the sediment was plated on 5% sheep blood agar, chocolate agar, Shaedler K-van agar, and Shaedler agar. Residual homogenate concentrate was inoculated in tryptic soy broth for enrichment. Inoculated plates were incubated at 37℃ in carbon dioxide (5% sheep blood and chocolate agar) or in anaerobiosis (Shaedler K-van and Shaedler agar). Inoculated solid media were incubated at 37℃ for 5 days. Colony-forming units on solid media were enumerated at 24 and 48 hours and after 5 days of incubation. Liquid media were checked daily for turbidity. In case of growth, subcultures for strain isolation were performed on the same solid media reported above. Isolates were identified by matrix assisted laser desorption ionization time-of-flight.
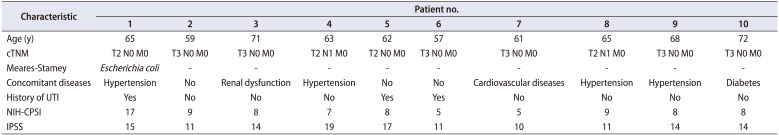
Ten consecutive patients were considered in this series (median age, 64 years; range, 57 to 72 years). The clinical characteristics of the patients are presented in Table 1. No patient reported LUTS. Only one patient had a positive result on a Meares-Stamey test for Escherichia coli. The remaining patients were negative for common bacteria and yeast and displayed no leukocyturia in any sample obtained from Meares-Stamey testing.
Two patients out of five (40%) showed bacterial growth on the microbiological analysis of the prostate core biopsy specimens. The following strains were isolated: Enterococcus faecalis, Enterococcus raffinosus, and Citrobacter freundii. One single biopsy showed growth of two microbial strains: E. faecalis and E. raffinosus. C. freundii was isolated from a different patient biopsy sample.
The SEM analysis revealed the presence of tissue calcifications in 6 of 10 (60%) of the analyzed biopsy specimens. Calcifications varied in size from a few to several hundred micrometers (Fig. 1A, B). High-magnification imaging of the calcification surface showed a crystalline structure (Fig. 1C) with protein residuals and a possible extracellular polymeric matrix from the microbial biofilm. The crystal composition varied among the patients, showing the presence of calcium and phosphorus or calcium, phosphorus, and magnesium. Round structures with a smooth surface and an average diameter ranging from 10 to 50 µm and morphologically compatible with corpora amylacea were also found in some biopsy specimens (Fig. 2A, B). Several intratissual aggregates of microorganisms with coccoid morphology with an extracellular polymeric matrix were clearly identified in 1 of 10 (10%) of the analyzed biopsy specimens (Fig. 2C). Two additional samples showed areas with micromorphology compatible with a microbial biofilm, but artifacts due to sample collection and preparation did not allow for a precise association with microorganisms.
In our series of patients examined by SEM, we clearly identified the presence of intratissual microbial biofilms in prostate core tissue. The same patients had positive cultures for E. faecalis and E. raffinosus from prostate core biopsy specimens, thus confirming the SEM findings of coccoid microbial biofilms.
Although prostatic calculi have long been recognized [16,17], and the relationship of urinary infections to the development of prostatic calculi and carcinoma has already been proposed [18,19], this study is the first to perform ultrastructural and microbiological analyses of prostate calcifications. Our findings suggest a significant role of bacterial biofilms in the genesis and development of these calcifications. A previous study from Dessombz and coworkers that used SEM and infrared spectroscopy on 23 prostatic stones revealed a high occurrence of bacterial imprints, revealing a past or present infection of the prostate tissue [20]. Inflammation induced by an infection may lead to cancerization of the tissue. However, no direct observation of microorganisms organized in microcolonies embedded in the extracellular matrix has been reported. To our knowledge, no relationship between a specific bacterial strain and prostate calcification has been reported in the literature so far. The results of this study may have several clinical implications, which are addressed below.
The role of bacterial biofilms is well known in urological infections, in particular, in chronic bacterial prostatitis [9,21]. Several authors have demonstrated the relationship between bacterial biofilms and antibiotic-resistant E. coli strains causing relapse of symptoms [16]. Antibiotic treatment is often unable to eradicate a microbial biofilm structure formed by strong biofilm-producer microorganisms, although the same antibiotic treatment is effective on planktonic bacteria [9,22,23]. In a cohort of chronic prostatitis patients treated with fluoroquinolones, Bartoletti and coworkers reported that 58.6% had negative microbiological tests after 3 months of antibiotic treatment [9]. However, only 16% of those patients reported a long-term improvement of symptoms [8]. This is in accordance with the fact that microbial biofilms harbor bacteria that can tolerate antibiotic treatment, the so-called “biofilm persister cells” [22,23]. Despite negative microbiological tests, persister cells can reconstitute the biofilm, thus inducing the reoccurrence of symptoms in patients with chronic bacterial prostatitis.
Although the present study findings were limited by the relatively small number of enrolled patients, this study should be regarded as an important pilot investigation for defining microbiological and ultrastructural characterization of prostate biopsy specimens. Our findings could thus be of value in defining future larger investigations. The combination of ultrastructural and cultural techniques applied to tissue samples obtained from the same subject indeed has the advantage of isolating and identifying the microorganisms associated with the prostatic tissue. Furthermore, this method allowed us to obtain direct information about their presence at the calcification surface in the aggregated form on the microbial biofilm. Moreover, possible contamination during tissue sampling could be excluded if biofilm architecture observed by SEM is typical of a mature biofilm embedded in the extracellular matrix. On the contrary, SEM has low sensitivity in detecting microbial biofilms within prostatic tissue. While high-resolution imaging is granted by using SEM, the identification of biofilms within the complex microscopic structure of the tissue biopsy limits the sensitivity of the overall technique. In addition, tissue sampling and processing can introduce morphological artifacts that complicate the identification of microorganisms, especially when noncoccoid species are present. This could explain why we were not able to identify microbial biofilms using SEM in the tissue of the patient with the biopsy culture positive for C. freundii. Further multicenter studies should be planned to better elucidate the intriguing role of bacterial biofilms in the genesis of prostate calcifications with the aim of defining new and more effective therapeutic strategies.
From a clinical point of view, this study has several implications. First, the clinical role of prostate calcifications should be reconsidered. In light of the findings reported here, prostate calcifications are not only a sonographic sign of previous prostatitis but should be considered a locus for difficult-to-treat bacteria (organized in a biofilm and having the potential to persist after common antibiotic treatments) within the prostate tissue. It is well known that not all patients with sonographic demonstration of prostate calcifications report clinical symptoms, but several authors have demonstrated that the presence and grade of prostate calcifications is associated with worsening of symptoms [4,5,7]. Moreover, some authors have reported that prostate calcifications are associated with prostate inflammation and symptoms [15,24,25]. Even if the natural history of prostate calcification is still not completely understood, we have added new data suggesting that bacterial biofilms might play a key role in the genesis of prostate calcifications and in the persistence of symptoms in a non-negligible fraction of antibiotic-treated patients. The presence of a bacterial biofilm represents a chronic inflammatory stimulus that could lead to the development of symptoms related to the grade of inflammation and the immune response of the patients. In the case of high-grade inflammation, the patient could report urinary or pelvic pain. The fluctuating symptomatology reported by the majority of patients might be explained by variation in the inflammatory response to the development and maturation of the bacterial biofilm. This hypothesis is supported by clinical observations that symptoms often decrease after antibiotic treatment but relapse after a variable period of time. The antibiotic treatment is probably effective in mitigating the grade of the infection but is not fully effective in eradicating the bacterial biofilm. Future studies should be designed to explore whether effective eradication of the bacterial biofilm could be associated with a good medium- and long-term clinical outcome of treatment.
Although supported by a limited number of patients, this study presented evidence of the validity of the analytical methods of integrating cultural and ultrastructural techniques for characterizing tissue obtained from prostatic resection and suggests a possible role of bacterial biofilm in the genesis of prostate calcifications and in the development of symptoms in chronic prostatitis.
References
1. Yang HJ, Huang KH, Wang CW, Chang HC, Yang TK. Prostate calcification worsen lower urinary tract symptoms in middle-aged men. Urology. 2013; 81:1320–1324. PMID: 23561714.

2. Suh JH, Gardner JM, Kee KH, Shen S, Ayala AG, Ro JY. Calcifications in prostate and ejaculatory system: a study on 298 consecutive whole mount sections of prostate from radical prostatectomy or cystoprostatectomy specimens. Ann Diagn Pathol. 2008; 12:165–170. PMID: 18486891.

3. Torres Ramírez C, Aguilar Ruiz J, Zuluaga Gómez A, del Río Sámper S, Issa Khozouz N. Structure of primary prostatic endogenous calculi. Arch Esp Urol. 1979; 32:581–590. PMID: 556206.
4. Sfanos KS, Wilson BA, De Marzo AM, Isaacs WB. Acute inflammatory proteins constitute the organic matrix of prostatic corpora amylacea and calculi in men with prostate cancer. Proc Natl Acad Sci U S A. 2009; 106:3443–3448. PMID: 19202053.

5. Geramoutsos I, Gyftopoulos K, Perimenis P, Thanou V, Liagka D, Siamblis D, et al. Clinical correlation of prostatic lithiasis with chronic pelvic pain syndromes in young adults. Eur Urol. 2004; 45:333–337. PMID: 15036679.

6. Klimas R, Bennett B, Gardner WA Jr. Prostatic calculi: a review. Prostate. 1985; 7:91–96. PMID: 3909127.

7. Shoskes DA, Lee CT, Murphy D, Kefer J, Wood HM. Incidence and significance of prostatic stones in men with chronic prostatitis/chronic pelvic pain syndrome. Urology. 2007; 70:235–238. PMID: 17826477.

8. Kanamaru S, Kurazono H, Terai A, Monden K, Kumon H, Mizunoe Y, et al. Increased biofilm formation in Escherichia coli isolated from acute prostatitis. Int J Antimicrob Agents. 2006; 28:S21–S25. PMID: 16828264.

9. Bartoletti R, Cai T, Nesi G, Albanese S, Meacci F, Mazzoli S, et al. The impact of biofilm-producing bacteria on chronic bacterial prostatitis treatment: results from a longitudinal cohort study. World J Urol. 2014; 32:737–742. PMID: 23918259.

10. Giubilei G, Mondaini N, Crisci A, Raugei A, Lombardi G, Travaglini F, et al. The Italian version of the National Institutes of Health Chronic Prostatitis Symptom Index. Eur Urol. 2005; 47:805–811. PMID: 15925077.

11. Badía X, García-Losa M, Dal-Ré R. Ten-language translation and harmonization of the International Prostate Symptom Score: developing a methodology for multinational clinical trials. Eur Urol. 1997; 31:129–140. PMID: 9076454.
12. Mazzoli S, Cai T, Rupealta V, Gavazzi A, Castricchi Pagliai R, Mondaini N, et al. Interleukin 8 and anti-chlamydia trachomatis mucosal IgA as urogenital immunologic markers in patients with C. trachomatis prostatic infection. Eur Urol. 2007; 51:1385–1393. PMID: 17107749.

13. Cai T, Wagenlehner FM, Mazzoli S, Meacci F, Mondaini N, Nesi G, et al. Semen quality in patients with Chlamydia trachomatis genital infection treated concurrently with prulifloxacin and a phytotherapeutic agent. J Androl. 2012; 33:615–623. PMID: 21979301.

14. Cai T, Caola I, Tessarolo F, Piccoli F, D'Elia C, Caciagli P, et al. Solidago, orthosiphon, birch and cranberry extracts can decrease microbial colonization and biofilm development in indwelling urinary catheter: a microbiologic and ultrastructural pilot study. World J Urol. 2014; 32:1007–1014. PMID: 24092275.

15. Cai T, Tiscione D, Caola I, Tessarolo N, Mondaini F, Meacci G, et al. Prostate calcifications and LUTS: the potential role of bacterial biofilm and prostate inflammation. Eur Urol Suppl. 2014; 13:e561.
17. Finkle AL. The relationship of antecedent genito-urinary infections to the development of prostatic calculi and carcinoma. Bull N Y Acad Med. 1953; 29:585–586. PMID: 13051704.
18. Eykyn S, Bultitude MI, Mayo ME, Lloyd-Davies RW. Prostatic calculi as a source of recurrent bacteriuria in the male. Br J Urol. 1974; 46:527–532. PMID: 4609168.

19. Meares EM Jr. Infection stones of prostate gland. Laboratory diagnosis and clinical management. Urology. 1974; 4:560–566. PMID: 4215187.
20. Dessombz A, Méria P, Bazin D, Daudon M. Prostatic stones: evidence of a specific chemistry related to infection and presence of bacterial imprints. PLoS One. 2012; 7:e51691. PMID: 23272143.

21. Soto SM, Smithson A, Martinez JA, Horcajada JP, Mensa J, Vila J. Biofilm formation in uropathogenic Escherichia coli strains: relationship with prostatitis, urovirulence factors and antimicrobial resistance. J Urol. 2007; 177:365–368. PMID: 17162092.

22. Lewis K. Multidrug tolerance of biofilms and persister cells. Curr Top Microbiol Immunol. 2008; 322:107–131. PMID: 18453274.

23. Donlan RM, Costerton JW. Biofilms: survival mechanisms of clinically relevant microrganisms. Clin Microbiol Rev. 2002; 15:167–193. PMID: 11932229.
24. Kim WB, Doo SW, Yang WJ, Song YS. Influence of prostatic calculi on lower urinary tract symptoms in middle-aged men. Urology. 2011; 78:447–449. PMID: 21689847.

25. Kwon YK, Choe MS, Seo KW, Park CH, Chang HS, Kim BH, et al. The effect of intraprostatic chronic inflammation on benign prostatic hyperplasia treatment. Korean J Urol. 2010; 51:266–270. PMID: 20428430.

Fig. 1
(A) Prostate core biopsy specimen showing several calcifications (brighter areas) within the tissue (darker areas). (B) Calcifications varied in size from a few to several hundred micrometers. (C) At high magnification, the calcification surface showed a crystalline structure with protein residuals and a possible extracellular polymeric matrix from the microbial biofilm. Scanning electron microscopy images were realized by collecting the signal from back-scattered electrons, giving compositional contrast between tissue and calcifications. (A) ×39, (B) ×500, (C) ×10,000.
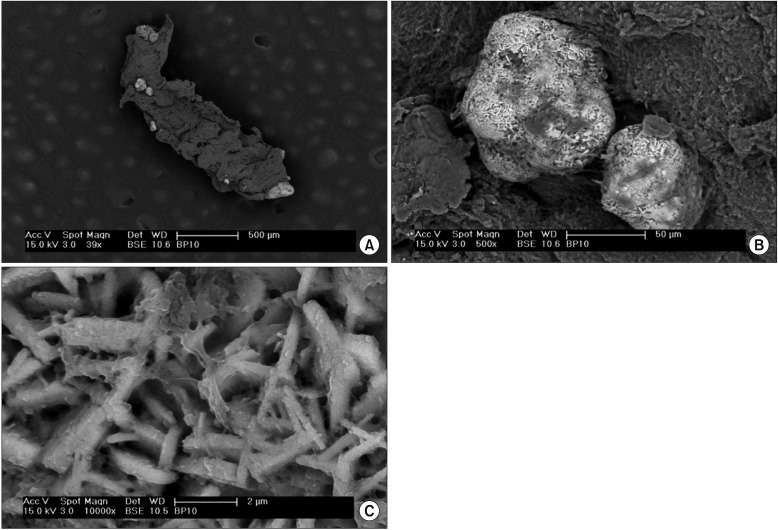
Fig. 2
(A) Prostate core biopsy specimen with calcifications and corpora amylacea (brighter areas) within the tissue (darker areas). (B) High-magnification details of some round-shaped structures, morphologically compatible with corpora amylacea. (C) Intratissual aggregate of microorganisms with coccoid morphology with extracellular polymeric matrix (microbial biofilm). Scanning electron microscopy images were realized by collecting the signal from back-scattered electrons (A) or secondary electrons to have the highest morphological detail. (A) ×100, (B) ×2,000, (C) ×8,000.
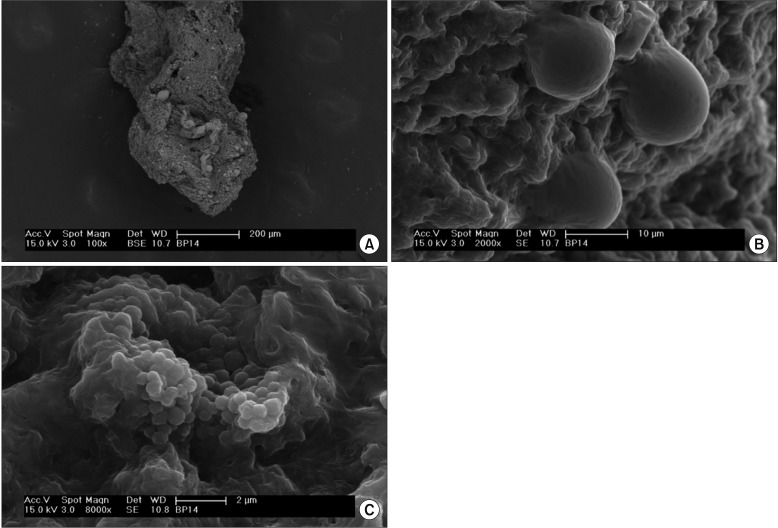
Table 1
Clinical and pathological characteristics of the enrolled patients




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download