Abstract
Background
Angiomatous meningioma is a rare histological subtype of meningioma. Therefore, this specific medical condition is rarely reviewed in the literature. In the present work, we report the clinical and radiological features with postoperative outcomes of angiomatous meningioma.
Methods
This retrospective study included the patients who were pathologically diagnosed with angiomatous meningioma after surgical resection between February 2010 and September 2015 in our institute. We analyzed the clinical data, radiological manifestation, treatment and prognosis of all patients.
Results
The 15 patients (5 males and 10 females) were diagnosed with angiomatous meningioma during the study period. The median age of patients at the time of surgery was 63 years (range: 40 to 80 years). According to Simpson classification, 7, 5, and 3 patients achieved Simpson grade I, II, and IV resection, respectively. In the follow-up period, recurrence was noted in one patient. Ten out of the 15 patients showed homogeneous enhancement. Two patients demonstrated cystic changes. There was no occurrence of calcification or hemorrhage in our patients. Characteristically, 14 out of 15 patients showed signal voids of vessels. Significant peritumoral edema was observed in the majority of tumors (67%).
Meningiomas are the most common type of tumor that make up about 20–30% of primary brain tumors with an adjusted annual incidence of about 4.5 per 100,000 individuals [1]. Although few meningiomas exhibit rapid growth, most of the meningiomas are slow-growing benign lesions. Meningiomas can be classified into various subtypes. According to the World Health Organization (WHO) classification of the tumors of the central nervous system, subtypes of meningioma are graded as grade I, II and III based on their histological characteristics [2].
About 80% of all meningiomas belong to WHO grade I, which includes meningothelial, fibrous, transitional, psammomatous, angiomatous, microcystic, secretory, metaplastic meningioma and lymphoplasmacyte-rich meningioma [34]. In addition to WHO grade I, atypical, chordoid and clear cell subtypes of meningiomas are classified as WHO class II. Furthermore, rhabdoid, papillary and anaplastic meningioma which demonstrate aggressive growth pattern with high recurrence rate are categorized as WHO class III [34].
Although grade I subtype constitutes the highest portion of all meningiomas, angiomatous meningioma is of very rare. Therefore, angiomatous meningioma is rarely reviewed. In this study, we present our clinical experiences with angiomatous meningioma patients.
This study included the patients, who underwent surgical resection and were pathologically diagnosed with angiomatous meningioma between February 2010 and September 2015 at our institute.
During the study period, 15 cases of angiomatous meningioma were identified. We retrospectively reviewed clinical data, radiological findings, treatment outcomes and prognosis of all patients in detail. The study protocol was reviewed and approved by the Institutional Review Board of our institute (SMC 2016-05-001), and adhered to the recommendations of the Declaration of Helsinki for biomedical research involving human subjects (1975).
All patients underwent preoperative magnetic resonance imaging (MRI) of brain. We analyzed preoperative MRI findings of 15 patients. After surgical resection, postoperative brain MRI was taken while in hospital or at the time of follow-up period. During follow-up period, brain MRI was regularly checked at intervals from six months to two years depending on the patient's status. Extent of resection was categorized according to the Simpson classification [5]. The histological diagnosis of angiomatous meningioma was confirmed by neuropathologist. Appearance of new enhancement around the resection margin and growth over than 25% in volume on follow-up MRI were regarded as recurrence.
The study group comprised of 5 men and 10 women, and their median age at the time of surgery was 63 years (range: 40 to 80 years).
The chief complaints of the patients were diverse in accordance with tumor location and size. Headache, seizure attack, weakness of limbs, memory impairment, facial nerve palsy and facial pain were representative symptoms. Four patients were incidentally detected during health check-up; they underwent operations because of their mass effect.
The duration between the onset of symptoms and admission ranged from 1 day to 2 years. One patient initially was treated by gamma knife radio-surgery. However the size of tumor was increased during follow-up period. Subsequently, the patient underwent operation 17 months after gamma knife radio-surgery.
The main location of angiomatous meningioma was cerebral convexity and other locations included sphenoid wing, sellar region, cerebellopontine angle (CPA), and tentorium of cerebellum. According to Simpson classification, 7, 5, and 3 patients were categorized as Simpson grade I, II, and IV resection, respectively. Complications occurred in three patients. One patient had tinnitus, hearing difficulty and facial nerve palsy after resection of the tumor in the CPA region. Another patient developed anosmia after surgical removal of the tumor located in the region of the tuberculum sellae. And last patient experienced left-sided hemiparesis after surgery. The summary of the patients with angiomatous meningioma is presented in Table 1.
We classified the lesions according to tumor size, shape, enhancement pattern, signal voids of vessel, dural tail sign and brain edema on preoperative MRI. All patients had enhancing solitary mass and the maximal diameter of the tumor ranged from 2.2 to 6.9 cm. The lesions were most commonly found at cerebral convexity area and demonstrated oval shaped morphology (47%). Ten out of 15 patients showed homogeneous enhancement while 5 patients showed heterogeneous enhancement. Two patients demonstrated cystic changes in tumor and 11 patients showed obvious dural tail sign. Calcification or hemorrhage was not observed in our patients. Fourteen out of 15 patients showed signal voids of vessels.
Significant peritumoral edema (bandwidth ≥2 cm) was observed in the majority of tumors (67%). Radiological characteristics of our cases are summarized in Table 2.
The duration of follow-up in our patients ranged from 6 to 50 months with a median of 17 months. Three patients were found to have residual mass after surgery (Simpson grade IV). Among them, one patient received gamma knife radio-surgery on residual mass. During the follow-up period, the residual tumor was in well controlled state. Another two patients showed no significant interval change on residual tumor in the left sphenoid wing on follow-up brain MR images without adjuvant therapy. Until now, none of our patients has died. However, there was only one case of recurrence in our series. Therefore, the recurrence rate was estimated to be 7% during the study period.
A 53-year-old woman visited our hospital presenting with seizure attack. The patient's brain MRI showed a well enhancing mass in left frontal convexity (Fig. 1A, B). Surgical resection for a frontal mass was performed. Simpson grade I could be achieved and gross total removal was identified based on patient's postoperative brain image. Postoperative period was uneventful and the neuropathologist reported the tumor as angiomatous meningioma (Fig. 1C). During 14 months from surgery, she had no seizure attack and her follow-up image showed no evidence of recurrence (Fig. 1D).
A 76-year-old man presents with 3 months old history of weakness of left lower extremity. The brain MRI showed solid and cystic mass in right frontal convexity (Fig. 2A). The signal void of vessel in the mass was not observed and significant brain edema was detected (Fig. 2B). Surgical resection with Simpson grade I was achieved and immediate post-operative period was not eventful. The brain tumor was classified as angiomatous meningioma in pathological examination (Fig. 2C). Postoperative brain MRI was undertaken on subsequent 3rd month demonstrated no residual mass (Fig. 2D) His neurologic symptom was gradually improved. However, the recurrence was observed on follow-up image after 13 months from surgery (Fig. 2E). The lesion was annually monitored. Lastly, 5-year follow-up MRI after operation showed that tumor was enlarged in inferior frontal gyrus (Fig. 2F). We recommended gamma knife radio-surgery, but the patient wanted regular follow-up observation. It has been 75 months post-surgery with no evident changes in neurological symptoms; furthermore, the patient is being evaluated on a regular basis.
Angiomatous meningioma is a rare subgroup of meningiomas. According to a reported study, only 38 (2.1%) angiomatous meningiomas from a total of 1,809 meningiomas have been identified [6]. Henceforth, there exists, scarce information regarding clinical and radiological characteristics of angiomatous meningioma. In fact, there are only a few case reports about angiomatous meningiomas and clinical studies regarding this issue are very rare. To the best of our knowledge, there are exist only two series about clinical features of angiomatous meningiomas [67].
The two previously reported studies demonstrate some distinct clinical characteristics of intracranial angiomatous meningiomas. Though the male to female ratio for meningioma in general is 0.50 (1:2), angiomatous meningioma showed much higher predominance in males [8]. Hasselblatt et al. [6] reported the male to female ratio as 0.73 (16:22) and Liu et al. [7] showed the male to female ratio as 1.08 (14:13) in their respective studies. However, in our current study, no male predominance (0.50) with respect to angiomatous meningioma was noted as compared with meningioma in general. This result may be attributed to small sample size of each series. Therefore, further studies are necessitated in this aspect.
Histopathological examination reveals distinctive characteristics of angiomatous meningiomas. Angiomatous meningioma shows abundance of well-formed vascular channels, sinusoids or capillaries prevailing on the background of an otherwise typical meningioma [9]. Considering the above-mentioned aspect, highly vascularized meningioma should be distinguished from both hemangiopericytoma and hemangioblastoma. However, angiomatous meningiomas focally exhibit classic meningothelial morphology; therefore, differential diagnosis becomes a difficult task [7]. Due to these pathological characteristics, angiomatous meningiomas may show some radiological features.
Basically, there are no other additional radiological features based on which angiomatous meningioma can be discriminated from other sub-classifications of meningiomas. However, angiomatous meningioma in spite of belonging to WHO grade I often shows distinguished perilesional edema. Tamiya et al. [10] perceived that angiomatous meningioma as well as meningothelial, anaplastic and microcystic histologic subtypes showed higher edema than other histologic variants. In conventional meningioma, venous obstruction, pial-meningeal anastomoses, increased capillary permeability, sex hormones and their receptors in meningiomas, and the secretion of vascular endothelial growth factor (VEGF) contribute to peritumoral edema [711121314]. In angiomatous meningioma, it is hypothesized that increased capillary permeability due to hypervascularity and VEGF secretion may mainly affect brain edema [7]. This is consistent with brain MR images revealing prominent signal voids in angiomatous meningiomas [7]. Our current study also supports the reported view. Most of our patients (93%) demonstrated signal voids in the tumor on previous brain MRI. Though homogenous enhancement on MRI has been mentioned as specific finding in angiomatous meningioma in a previous study, the data from our study was not remarkable [7]. Moreover, obvious dural tail sign rate in our study is of more common occurrence than previously reported study. Also, the observation in the present case study signified no noticeable calcification or hemorrhage and demonstrated cerebral convexity as most common tumor location with ten out of 15 patients illustrating the location. This ratio was analogous with results from a previous study [67].
WHO grade I meningiomas have a favorable prognosis. Since angiomatous meningioma belongs to this group, it has a similar behavior. In a recent study, overall tumor recurrence rates of WHO grade I meningioma for Simpson resection Grades I, II, III, and IV were 5%, 22%, 31%, and 35%, respectively [15]. In addition, WHO grade I meningioma have good prognosis after gross total resection. The recurrence rates of WHO Grade I convexity meningiomas are quite small (0.4%) after complete resection [15]. Our study also demonstrated good prognosis of angiomatous meningioma after gross total resection. Of the 15 patients, only one patient showed recurrence during follow-up period. In addition, three cases of residual tumor did not show progression of remnant tumor. However, our series involved a limited follow-up period. Given that angiomatous meningiomas belong to WHO grade I, a longer follow-up period may be required.
Angiomatous meningiomas are rare benign meningioma. Brain images of angiomatous meningioma usually show more signal void signs and brain edema than other meningioma. Angiomatous meningiomas have good prognosis after surgical resection, likewise other WHO grade I meningiomas. Based on the outcome of current case-study, we anticipate that our clinical information is becoming useful in diagnosis and treatment of angiomatous meningioma.
Figures and Tables
Fig. 1
MRI and histologic findings of case 4. A: The preoperative T1-weighted brain MRI with gadolinium enhancement demonstrates about 3 cm sized homogeneous enhancing mass. B: The preoperative T2-weighted brain MRI shows the signal voids of blood vessels. C: Histologic findings of angiomatous meningioma show predominence of vascular proliferations over the tumor cells. The vascular channels contain a mixture of delicate and thick walls with variable sizes (Hematoxylin and eosin stain, ×200). D: No recurrence is verified in the last follow-up images after 14 months from operation.
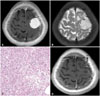
Fig. 2
MRI and histologic findings of case 15. A: The preoperative T1-weighted brain MRI with gadolinium enhancement shows a large cystic mass with mild midline shift. B: The preoperative T2-weighted brain MRI shows no definite signal voids of blood vessels and demonstrated significant brain edema. C: Histologic findings of angiomatous meningioma. In this case, most are small with hyalinized walls. Surrounding meningothelial cells have relatively uniform round to oval shape nuclei and nuclear pseudoinclusions with eosinophilic cytoplasm and indistinct cell borders (Hematoxylin and eosin stain, ×200). D: The postoperative T1-weighted MRI with gadolinium enhancement demonstrates that there is no residual mass at the tumorectomy site. E: The follow-up MRI after 13 months from surgery shows a small nodular enhancing lesion (T1-weighted with gadolinium enhancement). F: The latest follow-up MRI displays growth of recurrent tumor in inferior frontal gyrus (T1-weighted with gadolinium enhancement).
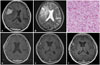
Table 1
Summary of the 15 angiomatous meningioma cases

Table 2
Comparative analysis between the current study and previous series

| Clinical characteristics and MRI findings | Present study [No. of cases (%)] | Liu et al., 2013 [No. of cases (%)] [7] | Hasselblatt et al., 2004 [No. of cases (%)] [6] |
|---|---|---|---|
| Age (median, range) (yr) | 63 (40–80) | 51.8* (24–72) | 64 (38–83) |
| Sex ratio (male:female) | 0.50 (5:10) | 1.08 (14:13) | 0.73 (16:22) |
| Tumor diameter (cm) | |||
| 3 | 3 (20) | 6 (22.2) | ND |
| 3–5 | 8 (53.3) | 13 (48.1) | ND |
| 5–7 | 4 (26.7) | 7 (25.9) | ND |
| ≥7 | 0 (0) | 1 (3.7) | ND |
| Tumor location | |||
| Convexity | 10 (66.7) | 18 (66.7) | 16 (42) |
| Falx, parasagittal | 7 (46.7) | 0 (0) | 13 (34) |
| Sphenoidal crest | 1 (6.7) | 4 (14.8) | 4 (11) |
| Cerebellopontine angle area | 2 (13.3) | 1 (3.7) | 4 (11)† |
| Tentorium of cerebellum | 1 (6.7) | 1 (3.7) | ND |
| Saddle area | 1 (6.7) | 2 (7.4) | ND |
| Petroclival | 0 (0) | 1 (3.7) | ND |
| Tumor shape | |||
| Oval | 7 (46.7) | 22 (81.5) | ND |
| Nodular lobulated | 2 (13.3) | 1 (3.7) | ND |
| Irregular | 6 (40) | 4 (14.8) | ND |
| Signal voids of vessel | 14 (93.3) | 27 (100) | ND |
| Tumor enhancement | |||
| Homogeneous | 10 (66.7) | 23 (85.2) | ND |
| Heterogeneous | 5 (33.3) | 4 (14.8) | ND |
| Cystic change | 2 (13.3) | 4 (14.8) | ND |
| Obvious meningeal tail sign | 11 (73.3) | 18 (66.7) | ND |
| Peritumoral edema | 10 (66.7) | ND | 26/35 (74) |
References
1. Riemenschneider MJ, Perry A, Reifenberger G. Histological classification and molecular genetics of meningiomas. Lancet Neurol. 2006; 5:1045–1054.

2. Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007; 114:97–109.

3. Robson DK. Pathology and genetics of tumours of the nervous system. In : Kleihues P, Cavenee WK, editors. The Journal of pathology. Lyon: IARC Press;2000. p. 314.
4. Tang H, Sun H, Chen H, et al. Clinicopathological analysis of metaplastic meningioma: report of 15 cases in Huashan Hospital. Chin J Cancer Res. 2013; 25:112–118.
5. Simpson D. The recurrence of intracranial meningiomas after surgical treatment. J Neurol Neurosurg Psychiatry. 1957; 20:22–39.

6. Hasselblatt M, Nolte KW, Paulus W. Angiomatous meningioma: a clinicopathologic study of 38 cases. Am J Surg Pathol. 2004; 28:390–393.
7. Liu Z, Wang C, Wang H, Wang Y, Li JY, Liu Y. Clinical characteristics and treatment of angiomatous meningiomas: a report of 27 cases. Int J Clin Exp Pathol. 2013; 6:695–702.
8. Roser F, Nakamura M, Ritz R, et al. Proliferation and progesterone receptor status in benign meningiomas are not age dependent. Cancer. 2005; 104:598–601.

9. Radner H, Blümcke I, Reifenberger G, Wiestler OD. [The new WHO classification of tumors of the nervous system 2000. Pathology and genetics]. Pathologe. 2002; 23:260–283.
10. Tamiya T, Ono Y, Matsumoto K, Ohmoto T. Peritumoral brain edema in intracranial meningiomas: effects of radiological and histological factors. Neurosurgery. 2001; 49:1046–1051. discussion 1051-2.

12. Pistolesi S, Fontanini G, Camacci T, et al. Meningioma-associated brain oedema: the role of angiogenic factors and pial blood supply. J Neurooncol. 2002; 60:159–164.
13. Domingo Z, Rowe G, Blamire AM, Cadoux-Hudson TA. Role of ischaemia in the genesis of oedema surrounding meningiomas assessed using magnetic resonance imaging and spectroscopy. Br J Neurosurg. 1998; 12:414–418.

14. Yoshioka H, Hama S, Taniguchi E, Sugiyama K, Arita K, Kurisu K. Peritumoral brain edema associated with meningioma: influence of vascular endothelial growth factor expression and vascular blood supply. Cancer. 1999; 85:936–944.
15. Nanda A, Bir SC, Maiti TK, Konar SK, Missios S, Guthikonda B. Relevance of Simpson grading system and recurrence-free survival after surgery for World Health Organization Grade I meningioma. J Neurosurg. 2016; 04. 08. [Epub]. DOI: 10.3171/2016.1.JNS151842.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download