Dear Editor:
Hailey-Hailey disease (HHD) is an autosomal dominant hereditary skin disease typically presenting with vesicles, erosions and crusts on the intertriginous areas such as the neck, axillae, groins, and perineum after the middle age. The responsible gene for HHD is ATP2C1, which encodes human secretory pathway Ca2+/Mn2+-ATPase protein 1 (SPCA1), a Ca2+ pump expressed in the Golgi apparatus1. Although over 150 pathological mutations have been identified throughout ATP2C1, no clear genotype-phenotype correlation has been revealed2.
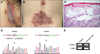
A 50-year-old Japanese male had a 2-year history of recurrent erythemas with vesicles and erosions on the posterior neck, axillae and popliteal fossae. Despite application of topical steroids, he had difficulty in walking due to painful inguinal erosions and was admitted. Skin lesions of the patient included erosions with pustular discharge on the inguinal area and erythemas with vesicles and crusts on the back, abdomen, axillae and thighs (Fig. 1A, B). The serum level of C-reactive protein was elevated (2.9 mg/dl), and Streptococcus agalactiae was cultured from inguinal discharge. Histopathological examination of vesicular erythema on the back revealed a separation of keratinocytes (acantholysis) at the suprabasal layers of the epidermis, which gave the appearance of “dilapidated brick wall” (Fig. 1C). Direct immunofluorescence was negative and generalized HHD with secondary bacterial infection was suspected. The lesions were improved by staying calm with oral clarithromycin and olopatadine hydrochloride, as well as topical zinc oxide for inguinal lesions and difluprednate for other lesions. After discharge, skin lesions occasionally flared and temporal administration of oral steroid was required.
By genetic analysis of the patient's peripheral blood, a novel heterozygous c.1627G>T transition on exon 18 of ATP2C1, causing a premature termination (PT) at amino acid 543 (p.Gly543X), was identified and the diagnosis of HHD was confirmed (Fig. 1D). We recently reported that the severe phenotype of a case with a PT-causing mutation is possibly caused by nonsense-mediated mRNA decay3. To confirm this possibility in the present case, ATP2C1 mRNA expression was examined with reverse transcription-polymerase chain reaction (RT-PCR) using mRNA extracted from the biopsy specimen and ATP2C1-specific primer pairs which amplify an upstream portion of the mutation described elsewhere4. Biopsy specimen of the lesional skin from a patient with urticarial rash was simultaneously examined for the control. As a result, sufficient ATP2C1 mRNA expression was unexpectedly observed in our case as compared to the case of urticarial rash with intact epidermis (Fig. 1E). Aminoglycosides, which reportedly induce readthrough of pathogenic nonsense mutations, had never been applied topically or systemically in our case5. Even if the mutant ATP2C1 mRNA was sufficiently expressed, severe functional defect of the mutant SPCA1 is expected because Gly543 is located within the cytoplasmic ATP-binding domain and PT at this residue causes deletion of the following 350 amino acids containing the C-terminal 6 transmembrane domains3. These observations suggest that functional impairment, rather than reduced mRNA expression, contributes to the phenotype in our case. Further study should be required to clarify how expression of the mutant ATP2C1 mRNA is regulated and relates to the disease severity.
Figures and Tables
Fig. 1
(A) Erosive lesion on the inguinal area of the patient. (B) Erythematous lesion with vesicles and crusts on the back of the patient. (C) Separation of keratinocytes at the suprabasal layers of the epidermis observed in the lesional back skin (H&E, ×100). (D) Heterozygous c.1627G>T transition causing a premature termination (p.Gly543X) of the ATP2C1 gene identified by the genetic analysis of the patient′s peripheral blood. (E) Sufficient ATP2C1 mRNA expression in the lesional skin of the patient, comparable in positive control skin with intact epidermis from a patient with urticarial rash. Gene-specific primer pairs used for RT-PCR were as follows: 5′-CCTTATTATGCTGCTTCTGG-3′ and 5′-CTTTGCTTTGCCACATCTGA-3′ for ATP2C1, and 5′-CTCCATCATGAAGTGTGACG-3′ and 5′-TGCTTGCTGATCCACATCTG-3′ for β-actin, which was examined as an internal control.
ACKNOWLEDGMENT
We would like to thank Miss Yumi Nakatani and Miss Risa Tsuneyoshi for their excellent technical assistance. This work was supported in part by a grant from the Japan Ministry of Health, Labor and Welfare.
References
1. Hu Z, Bonifas JM, Beech J, Bench G, Shigihara T, Ogawa H, et al. Mutations in ATP2C1, encoding a calcium pump, cause Hailey-Hailey disease. Nat Genet. 2000; 24:61–65.

2. Dobson-Stone C, Fairclough R, Dunne E, Brown J, Dissanayake M, Munro CS, et al. Hailey-Hailey disease: molecular and clinical characterization of novel mutations in the ATP2C1 gene. J Invest Dermatol. 2002; 118:338–343.

3. Matsuda M, Hamada T, Numata S, Teye K, Okazawa H, Imafuku S, et al. Mutation-dependent effects on mRNA and protein expressions in cultured keratinocytes of Hailey-Hailey disease. Exp Dermatol. 2014; 23:514–516.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download