Abstract
Purpose
To investigate the characteristics and the results of long-term follow-up of limbal epithelial cells cultivated in vivo on amniotic membranes (LIVAM) in corneal limbal deficiency.
Methods
Twenty-two eyes of twenty-two patients diagnosed with corneal limbal deficiency underwent transplantation of in vivo cultivated corneal limbal epithelial cells on the amniotic membrane. Biopsy and immunohistochemical staining (AE5, MUC5AC) of the amniotic membrane cultivated for one week were performed to verify that the cultivated epithelial cells on the amniotic membrane were corneal epithelial cells. Impression cytology was performed to evaluate the characteristics of the transplanted corneal limbal epithelial cells at postoperative 1 week, 3 months, 6 months, and 1 year.
Results
Successful epithelial growth was observed on the amniotic membrane at one week. The epithelial cells were confirmed to be corneal epithelial cells by immunohistochemical staining. Transplanted in vivo cultivated corneal epithelial cells were confirmed to have corneal specificity by impression cytology and immunohistochemical staining at postoperative 1 week, 3 months, 6 months, and 1 year.
Conclusions
In vivo cultured corneal epithelial cells showed morphological and immunohistochemical findings similar to those of normal corneal epithelial cells. Transplanted in vivo cultivated corneal epithelial cells were maintained and showed the characteristics of corneal epithelial cells. Transplantation of in vivo cultivated corneal limbal epithelial cells can be performed to reconstruct the corneal limbus in treating corneal limbal deficiency.
References
1. Schermer A, Galvin S, Sun TT. Differentiation-related expression of a major 64K corneal keratin in-vivo and in culture suggests limbal location of corneal epithelial stem cells. J Cell Biol. 1986; 103:49–62.
2. Cotsarelis G, Cheng SZ, Dong G, et al. Existence of slow-cycling limbal epithelial basal cells that can be preferentially stimulated to proliferate: implications on epithelial stem cells. Cell. 1989; 57:201–9.

3. Shapiro MS, Friend J, Thoft RA. Corneal reepithelialization from the conjunctiva. Invest Ophthalmol Vis Sci. 1981; 21:135–42.
4. Tseng SC. Regulation and clinical implications of corneal epithelium stem cells. Mol Biol Rep. 1996; 23:47–58.
5. Kenyon KR, Tseng SC. Limbal autograft transplantation for ocular surface disorders. Ophthalmology. 1989; 96:709–23.

6. Tsai RJF, Li LM, Chen JK. Reconstruction of damaged corneas by transplantation of autologous limbal epithelial cells. N Engl J Med. 2000; 343:86–93.

7. Koizumi N, Fullwood NJ, Bairaktaris G, et al. Cultivation of corneal epithelial cells on intact and denuded human amniotic membrane. Invest Ophthalmol Vis Sci. 2000; 41:2506–13.
8. Koizumi N, Inatomi T, Suzuki T, et al. Cultivated corneal epithelial transplantation for ocular surface reconstruction in acute phase of Stevens-Johnson syndrome. Arch Ophthalmol. 2001; 119:298–300.
9. Koizumi N, Inatomi T, Suzuki T, et al. Cultivated corneal epithelial stem cell transplantation in ocular surface disorders. Ophthalmology. 2001; 108:1569–74.
10. Lee DW, Park WC. Transplantation of in vivo cultivated limbal corneal epithelial cells with total limbal stem cell deficiency. J Korean Ophthalmol Soc. 2005; 46:494–503.
11. Krachmer JH, Mannis MJ, Holland EJ. Cornea. 1st ed.1. St. Louis: Mosby;1997. p. 42–4.
12. Zieske JD, Bukusoglu G, Gipson IK. Enhancement of vinculin synthesis by migratory stratified squamous epithelium. J Cell Biology. 1989; 109:571–6.
13. Chung EH, Heutcheon AE, joyce NC, Zieske JD. Synchronization of the G1/S transition in response to corneal debridement. Invest Ophthalmol Vis Sci. 1999; 40:1952–8.
14. Hall PA, Watt FM. Stem cells: The generation and maintenance of cellular diversity. Development. 1989; 106:619–33.

15. Thoft RA, Friend J. The X,Y,Z hypothesis of corneal epithelial maintenance. Invest Ophthalmol Vis Sci. 1983; 24:1442–3.
16. Sandvig KU, Haaskjold E, Bjerknes R, et al. Cell kinetics of conjunctival and corneal epithelium during regeneration of different-sized corneal epithelial defects. Acta Ophthalmol. 1994; 172:43–8.

17. Yanoff M, Fine BS. Ocular pathology. 1st ed.1. Philadelphia: Harper and Row;1982. p. 287.
18. Pinkerton OD. Immunologic basis for the pathogenesis of pterygium. Am J Ophthalmol. 1984; 98:225–8.

19. Chun DH, Jeon SL, Lee JY, Choi TH. The effect of amniotic membrane transplantation on corneal epithelial cell prolifera-tinon. J Korean Ophthalmol Soc. 2002; 43:1746–57.
20. Park GS, Choi TH, Park WC, Kim JC. The clinical efficacy of amniotic membrane transplantation and limbal conjunctival autograft in patients with recurrent pterygium or pseudopterygium. J Korean Ophthalmol Soc. 2001; 42:1143–9.
21. Lee DY, Park WC, Rho SH. The effects of amniotic membrane ointment application on photorefractive keratectomized cornea in rabbits. J Korean Ophthalmol Soc. 2000; 41:2069–77.
22. Taylor RJ, Wang MX. Rate of reepithelialization following amniotic membrane transplantation. Invest Ophthalmol Vis Sci. 1998; 39:S1038.
23. Kim JS, Kim JC, Na BK, et al. Amniotic membrane patching promotes healing and inhibits proteinase activity on wound healing following acute corneal alkali burn. Exp Eye Res. 2000; 70:329–37.

24. Buultmann S, You L, Spandau U, et al. Amniotic membrane down-regulate chemokine expression in human keratocytes. Invest Ophthalmol Vis Sci. 1999; 40:S3044.
25. Pellegrini G, Traverso CE, Franzi AT, et al. Long-term restoration of damaged corneal surfaces with autologous cultivated corneal epithelium. Lancet. 1997; 349:990–3.

26. Koizumi N, Inatomi T, Quantock AJ, et al. Amniotic membrane as a substrate for cultivating limbal corneal epithelial cells for autologous trasplantation in rabbits. Cornea. 2000; 19:65–71.
27. Shimazaki J, Aiba M, Goto E, et al. Transplantation of human limbal epithelium cultivated on amniotic membrane for the treatment of severe ocular surface disorder. Ophthalmology. 2002; 109:1285–90.
Figure 1.
Schematic drawing of the orientation of each cellulose acetate paper applied on the ocular surface.
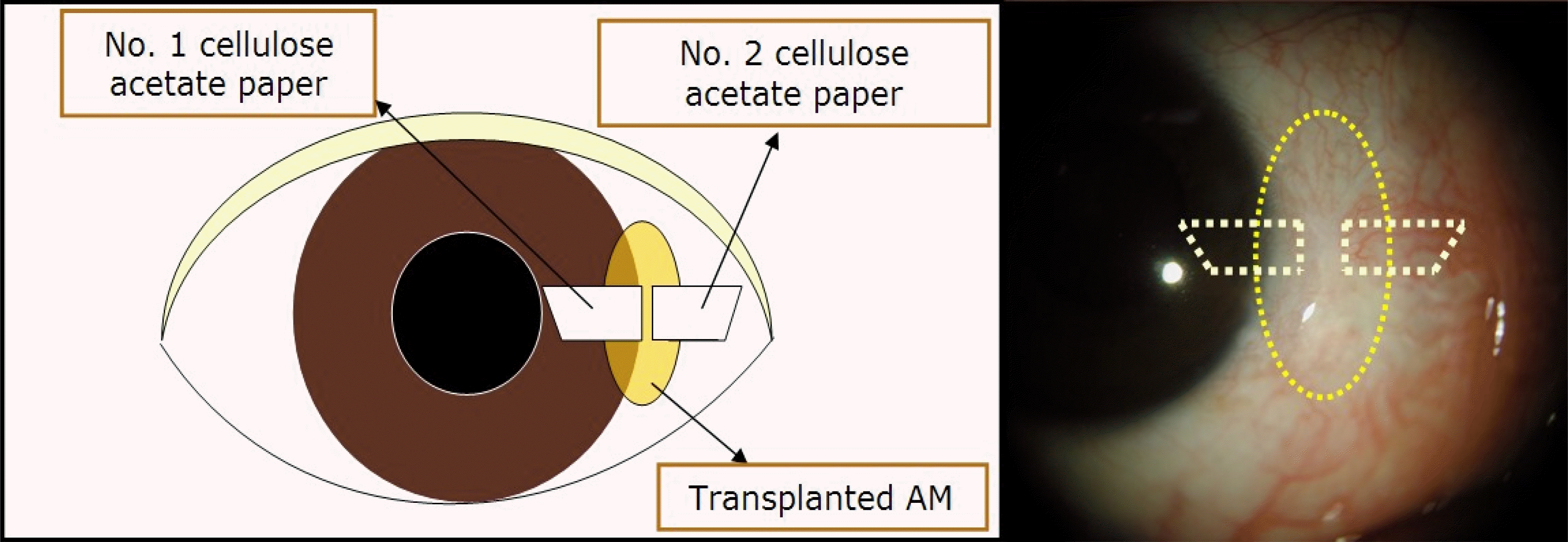
Figure 2.
(A) In vivo cultivated corneal epithelial cells on human amniotic membrane for a week. (Hematoxylin-Eosin stain, Original magnification ×100), (B) Periodic Acid-Schiff stain (original magnification ×100), (C) Anti-AE5 immunohistochemical stain (original magnification ×100). Light microscopy demonstrates well stratified and cuboidal corneal epithelial cells firmly attached to basement membrane of human amniotic membrane. There is no goblet cell in the PAS staining. In immunohistochemical staining, cultivated epithelial cells show positive staining for AE5.
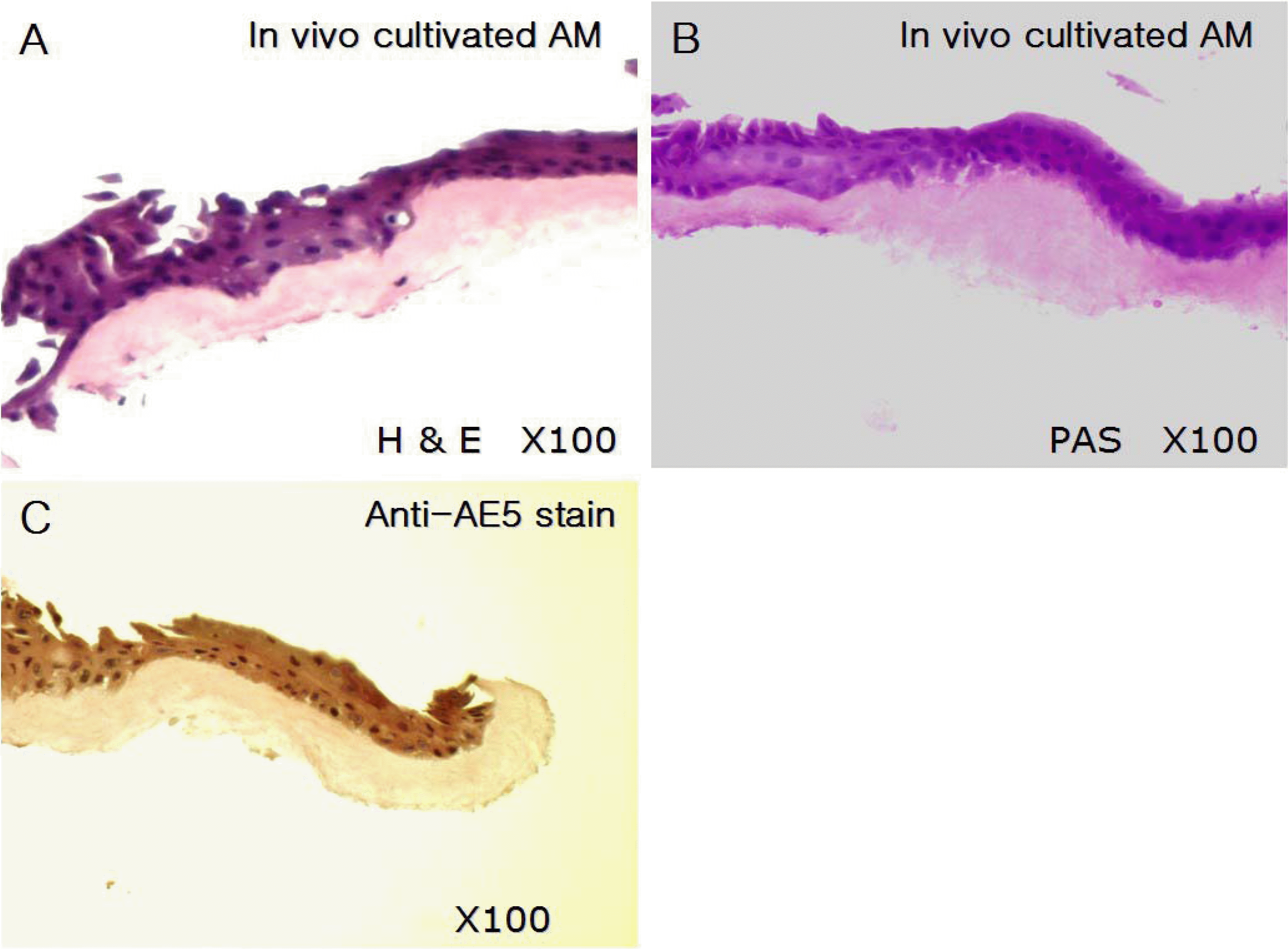
Figure 3.
Impression cytology from the cornea, transplanted amniotic membrane and conjunctiva at postoperative a week (original magnification ×100). PAS stain: abundant periodic acid-schiff negative epithelial cells on corneal and amniotic membrane side, but periodic acid-Schiff positive on the conjunctival side (arrow); Anti-AE5 immunohistochemical stain: positive staining on the corneal and amniotic membrane side; Anti-MUC5AC immunohistochemical stain: positive staining only on the conjunctival side.
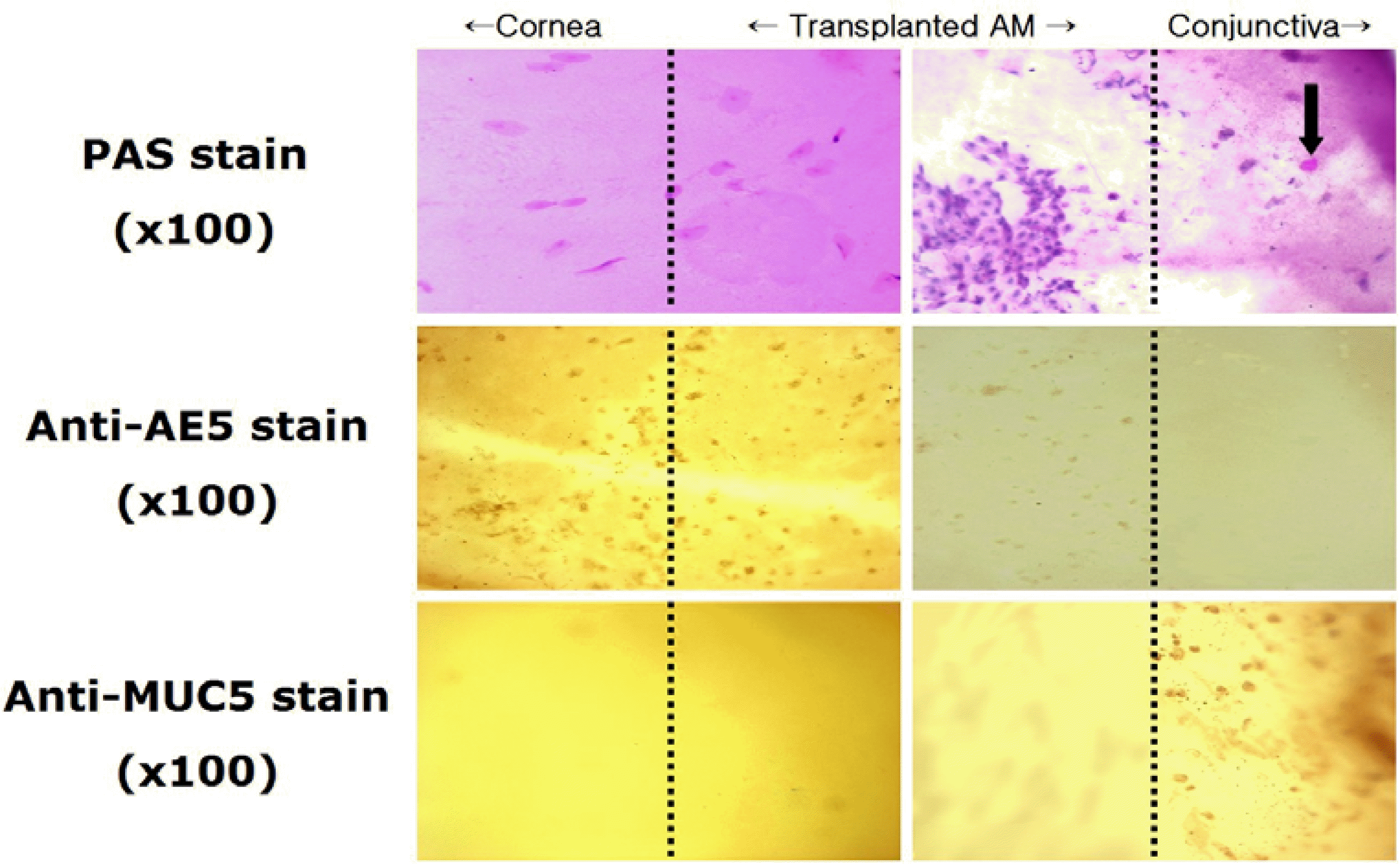
Figure 4.
Impression cytology from the cornea, transplanted amniotic membrane and conjunctiva at postoperative 3 months (original magnification ×100). PAS stain: abundant periodic acid-Schiff negative epithelial cells on the corneal and amniotic membrane side, but periodic acid-Schiff positive on the conjunctival side (arrow); Anti-AE5 immunohistochemical stain: positive staining on the corneal and amniotic membrane side; Anti-MUC5AC immunohistochemical stain: positive staining only on the conjunctival side.
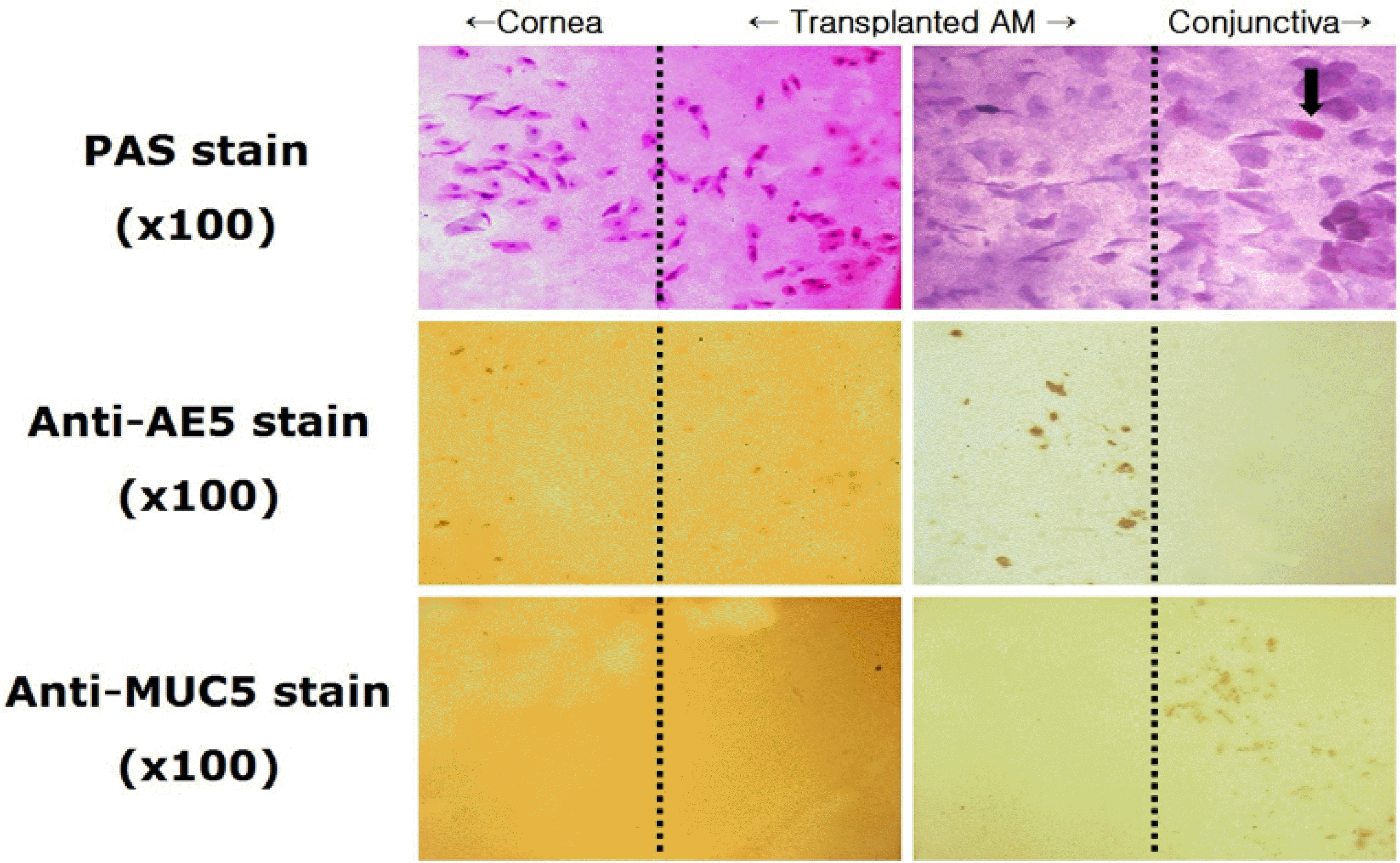
Figure 5.
Impression cytology from the the cornea, transplanted amniotic membrane and conjunctiva at postoperative 6 months (original magnification ×100). PAS stain: abundant periodic acid-Schiff negative epithelial cells on the corneal and amniotic membrane side, but periodic acid-Schiff positive on the conjunctival side (arrow); Anti-AE5 immunohistochemical stain: positive staining on the corneal and amniotic membrane side; Anti-MUC5AC immunohistochemical stain: positive staining only on the conjunctival side.
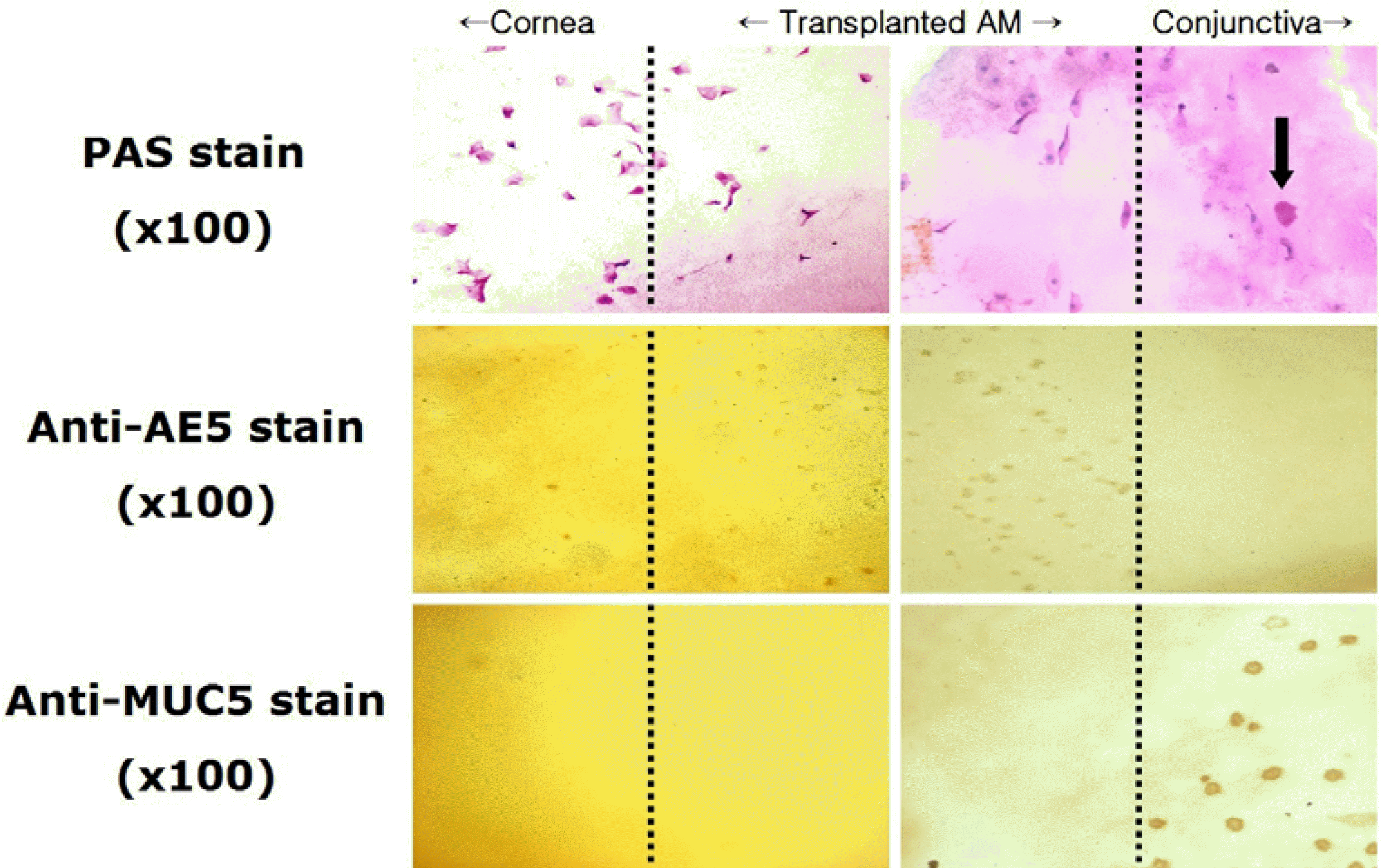
Figure 6.
Impression cytology from the cornea, transplanted amniotic membrane and conjunctiva of patient with scleral recurrence at postoperative 6 months (original magnification ×100). Anti-AE5 immunohistochemical stain: positive staining on the corneal and amniotic membrane side; Anti-MUC5AC immunohistochemical stain: positive staining on the transplanted amniotic membrane and conjunctival side.
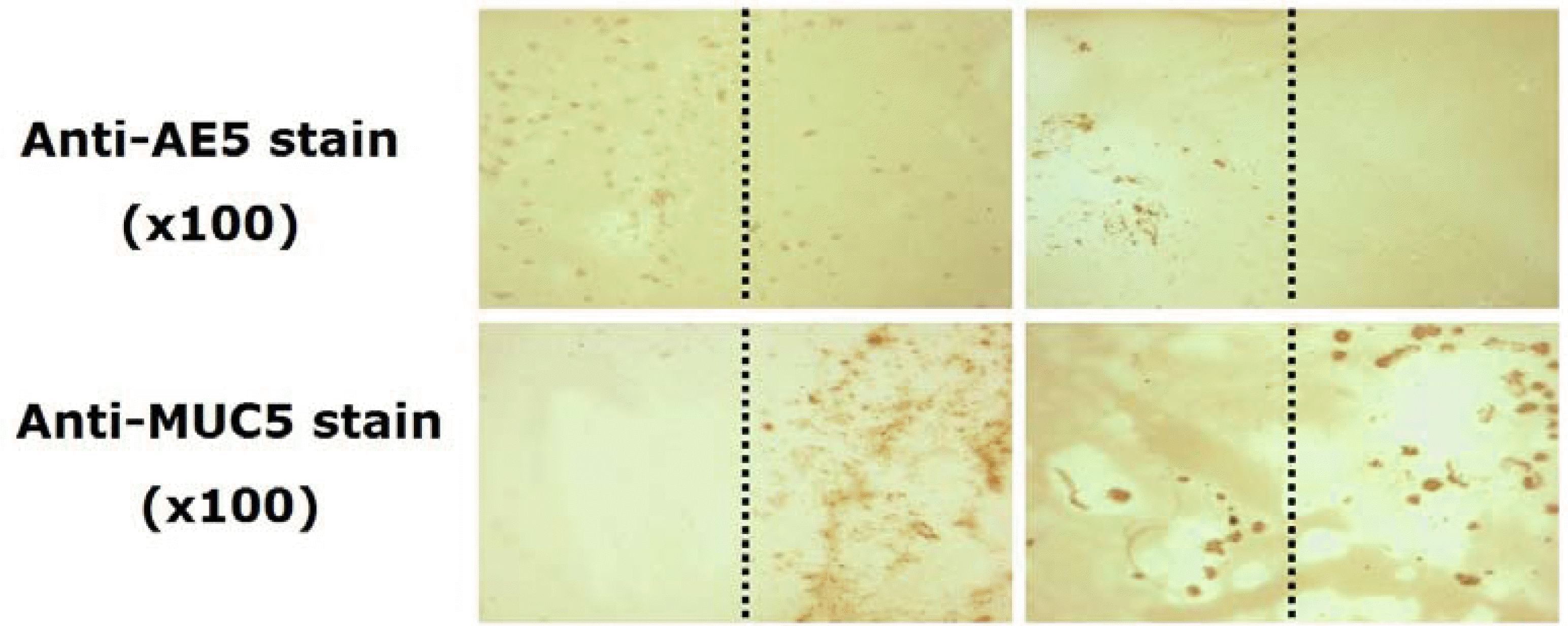
Figure 7.
Impression cytology from the cornea, transplanted amniotic membrane and conjunctiva at postoperative 1 year (original magnification ×100). PAS stain: abundant periodic acid-Schiff negative epithelial cells on the corneal and amniotic membrane side, but periodic acid-Schiff positive on the conjunctival side (arrow); Anti-AE5 immunohistochemical stain: positive staining on the corneal and amniotic membrane side; Anti-MUC5AC immunohistochemical stain: positive staining only on the conjunctival side.
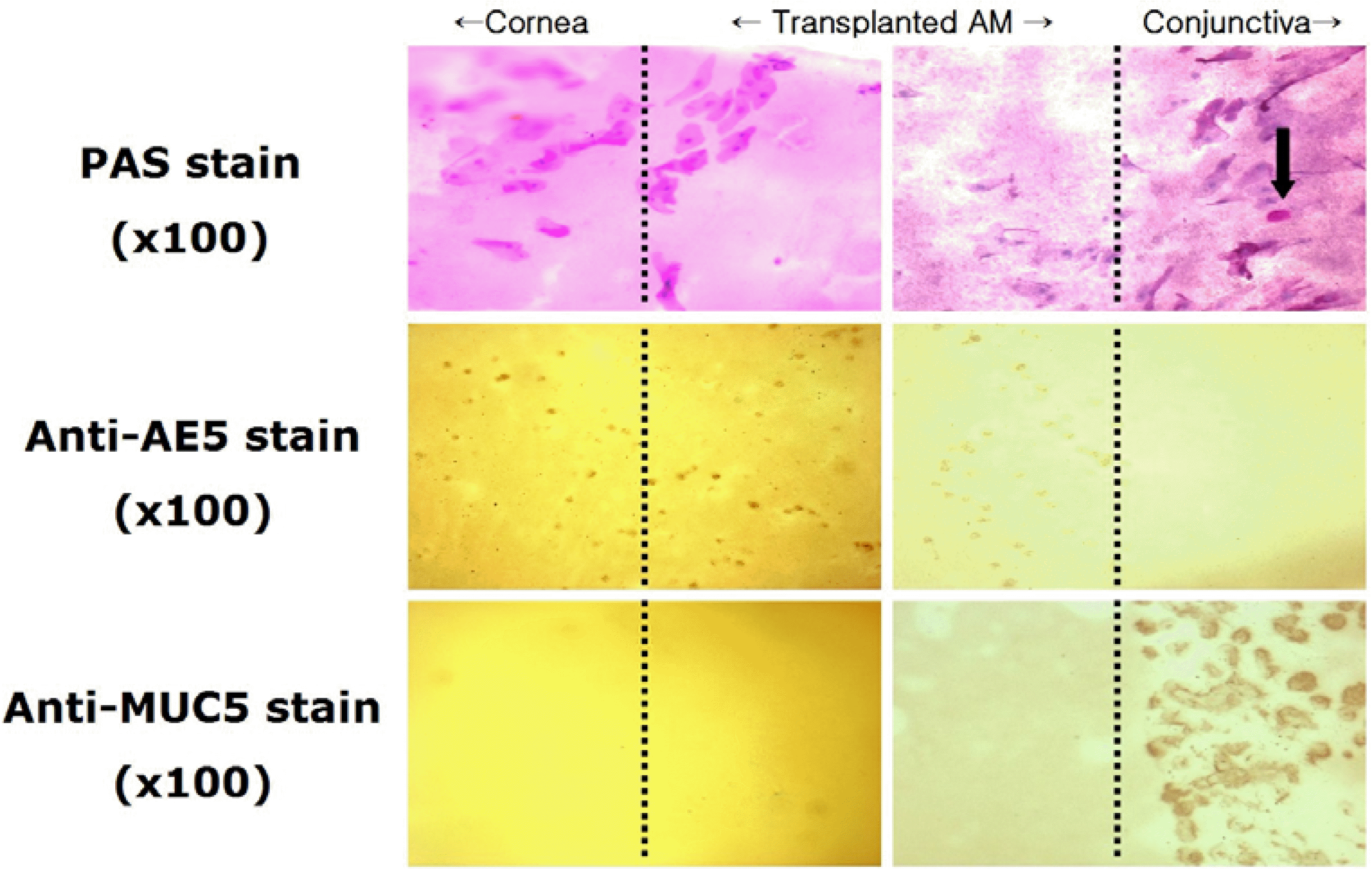
Table 1.
The results of immunohistochemistry of transplanted epithelial cells cultivated in vivo on the amniotic membrane




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download