This article has been
cited by other articles in ScienceCentral.
Abstract
Background
The purpose of this study was to determine the extent of air and surface contamination of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) in four health care facilities with hospitalized coronavirus disease 2019 (COVID-19) patients.
Methods
We investigated air and environmental contamination in the rooms of eight COVID-19 patients in four hospitals. Some patients were in negative-pressure rooms, and others were not. None had undergone aerosol-generating procedures. On days 0, 3, 5, and 7 of hospitalization, the surfaces in the rooms and anterooms were swabbed, and air samples were collected 2 m from the patient and from the anterooms.
Results
All 52 air samples were negative for SARS-CoV-2 RNA. Widespread surface contamination of SARS-CoV-2 RNA was observed. In total, 89 of 320 (27%) environmental surface samples were positive for SARS-CoV-2 RNA. Surface contamination of SARS-CoV-2 RNA was common in rooms without surface disinfection and in rooms sprayed with disinfectant twice a day. However, SARS-CoV-2 RNA was not detected in a room cleaned with disinfectant wipes on a regular basis.
Conclusion
Our data suggest that remote (> 2 m) airborne transmission of SARS-CoV-2 from hospitalized COVID-19 patients is uncommon when aerosol-generating procedures have not been performed. Surface contamination was widespread, except in a room routinely cleaned with disinfectant wipes.
Go to :

Graphical Abstract
Go to :

Keywords: SARS-CoV-2, Transmission, Environmental Sampling, Droplet
INTRODUCTION
Coronavirus disease 2019 (COVID-19) is a respiratory disease caused by the novel coronavirus severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). It was first detected in Wuhan, China in December 2019. Despite efforts to eliminate the disease, it remains a global health threat and has caused more than six million confirmed infections and 370,000 deaths as of June 1, 2020.
1 The basic reproductive number of SARS-CoV-2 is estimated to be between 1.95 and 6.49, necessitating aggressive control measures (e.g., active early surveillance and quarantine).
2
It is necessary to understand the modes of transmission of COVID-19 to develop effective control measures. Epidemiological studies have led the Centers for Disease Control and Prevention (CDC) to posit that person-to-person transmission of SARS-CoV-2 is mediated primarily by respiratory droplets or contact with contaminated surfaces.
3 However, remote (> 2 m) airborne transmission has been suggested with increasing frequency.
456789 In high-risk transmission settings, such as healthcare facilities, knowledge of the modes of transmission and adoption of the appropriate respiratory precautions are key factors in infection control. Studies of the environmental contamination associated with COVID-19 patients are needed to improve our understanding of the modes of transmission of SARS-CoV-2. However, few case reports are available.
1011
The objectives of the present study were 1) to investigate air and environmental contamination caused by COVID-19 patients in a variety of hospital settings; 2) to evaluate the effectiveness of environmental cleaning; and 3) to examine the potential for remote airborne transmission in the absence of aerosol-generating procedures.
Go to :

METHODS
Hospital characteristics
Eight COVID-19 patients who were not subjected to aerosol-generating procedures were enrolled from March 25 to April 8, 2020, at four facilities: Chonnam National University (CNU) Hospital (Hospital A; Gwangju, Korea), CNU Hwasun Hospital (Hospital B; Hwasun, Korea), CNU Bitgoeul Hospital (Hospital C; Gwangju, Korea), and Keimyung University Daegu Dongsan Hospital (Hospital D; Daegu, Korea). Room conditions differed by hospital. Hospitals A and B had seven and five designated airborne infection isolation rooms (AIIRs), respectively, with a minimum of 15 air changes per hour. Hospital C has 180 beds and is a designated COVID-19 hospital. Patients in Hospital C were admitted to isolation rooms without negative air pressure. Hospital D, which is located in an outbreak region, has 465 beds and is also designated for COVID-19 patients. Patients are cohorted in common rooms without negative air pressure. The three patients in Hospital D shared a room containing five beds. In all hospitals, the surfaces were disinfected before admission and after discharge. In addition, wipes for daily surface disinfection were used in Hospital B, and spray disinfectants were used twice a day in Hospital D. The wipes used for surface cleaning in Hospital B were ED Wipes (MH Healthcare, Gimpo, Korea) containing benzalkonium chloride 0.4% and four enzymes (protease, alpha-amylase, lipase, and cellulase).
Aerosol-generating procedures were defined as open suctioning of airways, sputum induction, cardiopulmonary resuscitation, endotracheal intubation and extubation, non-invasive ventilation, bronchoscopy, manual ventilation, nebulizer administration, and high flow O2 delivery, following the recommendations of the CDC.
Sampling and collection
All patients were admitted to the hospital within 7 days of the onset of respiratory symptoms. Air and surface samples were obtained four times per patient: before admission and on hospital days 3, 5, and 7. To exclude the possibility of droplet and droplet nucleus acquisition in short-distance during air collection, air flow was taken into account, and room air was sampled 2 m from the patient in the direction of air escape. The MD8 Airport Portable Air Sampler (Sartorius Stedim Biotech; Göttingen, Germany) with a gelatin membrane filter was used to collect 1,000 L air over 20 minutes at a rate of 50 L/minute. To obtain a positive control sample, a patient deposited saliva droplets directly onto the gelatin membrane filter. This produced a sample that was positive for SARS-CoV-2 RNA. Surfaces at 10–18 sites in the patients' rooms were sampled using wet cotton swabs immediately before the next scheduled daily surface cleaning. Each swab was used to sample an area of 0.25 m2. The entire surface of toilet door handles, patients' laptops, and patients' mobile phones were swabbed. The swabs were then submerged in 2 mL viral transport medium. Each sample was then individually wrapped and transported to the laboratory of CNU Hospital within 3 hours of sampling by a contracted car racer.
Laboratory procedures
Real-time reverse-transcription polymerase chain reaction (rRT-PCR) was performed in the laboratory of CNU Hospital, a diagnostic facility for COVID-19 authorized by the Korea CDC. First, 200 μL was taken from each sample, and RNA was extracted using an automated nucleic acid extraction system (AdvanSure™ E3 System; LG Chem, Seoul, Korea). The extracted RNA was amplified using a commercial rRT-PCR kit (PowerChek™ 2019-nCoV Real-time PCR Kit; KogeneBiotech, Seoul, Korea) and detection system (CFX96™ Real-time PCR detection system; Bio-Rad, Hercules, CA, USA) to detect the envelope (E) and RNA-dependent RNA polymerase (RdRP) genes. Forty PCR cycles were performed and cycle threshold (Ct) values were determined for each gene. For quality control, each test included positive, negative, and internal controls. A positive rRT-PCR result was defined as a Ct value of 35 or less, whereas a Ct value over 40 was considered negative; a Ct value between 35 and 40 was considered indeterminate. The figure shows the lower of the Ct values for the E and RdRP genes.
Ethics statement
Due to the nature of this study, the Institutional Review Board of Chonnam National University Hwasun Hospital reviewed and approved the research protocol and waived the requirement for informed consent (CNUHH-2020-163).
Go to :

RESULTS
We enrolled two patients from Hospital A (one with an upper respiratory infection and one with pneumonia), one patient with pneumonia from Hospital B, two patients from Hospital C (one with an upper respiratory infection and one with pneumonia), and three patients with pneumonia from Hospital D. The patient characteristics are described in
Table 1.
Table 1
Characteristics of eight patients with COVID-19 in this study
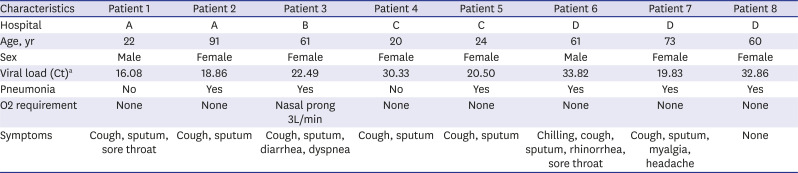
|
Characteristics |
Patient 1 |
Patient 2 |
Patient 3 |
Patient 4 |
Patient 5 |
Patient 6 |
Patient 7 |
Patient 8 |
|
Hospital |
A |
A |
B |
C |
C |
D |
D |
D |
|
Age, yr |
22 |
91 |
61 |
20 |
24 |
61 |
73 |
60 |
|
Sex |
Male |
Female |
Female |
Female |
Female |
Male |
Female |
Female |
|
Viral load (Ct)a
|
16.08 |
18.86 |
22.49 |
30.33 |
20.50 |
33.82 |
19.83 |
32.86 |
|
Pneumonia |
No |
Yes |
Yes |
No |
Yes |
Yes |
Yes |
Yes |
|
O2 requirement |
None |
None |
Nasal prong 3L/min |
None |
None |
None |
None |
None |
|
Symptoms |
Cough, sputum, sore throat |
Cough, sputum |
Cough, sputum, diarrhea, dyspnea |
Cough, sputum |
Cough, sputum |
Chilling, cough, sputum, rhinorrhea, sore throat |
Cough, sputum, myalgia, headache |
None |

SARS-CoV-2 RNA was not detected in any of the baseline environmental swabs taken prior to patient admission at the four hospitals. Following patient admission, SARS-CoV-2 RNA was detected in 89 of 330 (27%) environmental surface samples from the four hospitals. The rRT-PCR showed no SARS-CoV-2 RNA in the 32 air samples from the patients' rooms or in the 20 air samples from the five anterooms, regardless of room type (AIIR vs. non-AIIR).
In the rooms in Hospital A (AIIR without routine disinfection), SARS-CoV-2 RNA was detected in 52 of 108 (48%) surface samples. The surfaces sampled after patient admission included bed rails, medical carts, the floor, door handles, the bathroom sink, the toilet, and other fomites (e.g., cell phones, intercoms, and TV remote controllers) (
Fig. 1A). Despite extensive surface sampling, SARS-CoV-2 RNA was not detected in the room in Hospital B (AIIR with routine surface cleansing using disinfectant wipes). However, considerable viral RNA was detected in the patient's respiratory samples (Ct value 22.4–28.9) (
Fig. 1B). Detailed Ct values are shown in
Table 2. SARS-CoV-2 RNA was detected in 14 of 84 surface samples (17%) from Hospital C (isolation room without routine surface cleaning). Surfaces sampled included bathroom door handles, the floor, and other fomites (
Fig. 1C). SARS-CoV-2 RNA was detected in 23 of 90 surface samples (26%) from the five-bed common room in Hospital D. Surfaces sampled included bed rails, the floor, the bathroom sink, the toilet, and other fomites (
Fig. 1D).
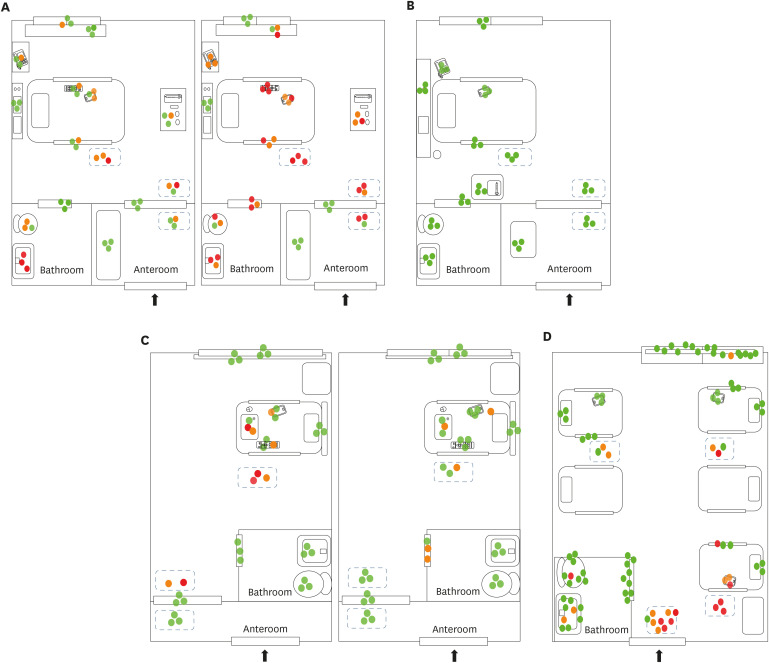 | Fig. 1
Environmental sampling sites and the Ct values of rooms in each hospital are illustrated in dots. Red dot indicates sample with Ct value ≤ 35 in real-time polymerase chain reaction; orange dot indicates sample with Ct value > 35 and ≤ 40; green dot indicates sample with negative result. Two AIIRs in Hospital A (A) and an AIIR in Hospital B (B). Two non-AIIRs in Hospital C (C) and a cohort non-AIIR in Hospital D (D).
Ct = cycle threshold, AIIR = airborne infection isolation room, Hospital A = Chonnam National University Hospital, Hospital B = Chonnam National University Hwasun Hospital, Hospital C = Chonnam National University Bitgoeul Hospital, Hospital D = Keimyung University Daegu Dongsan Hospital.

|
Table 2
Cycle threshold values of samples from patients and environments
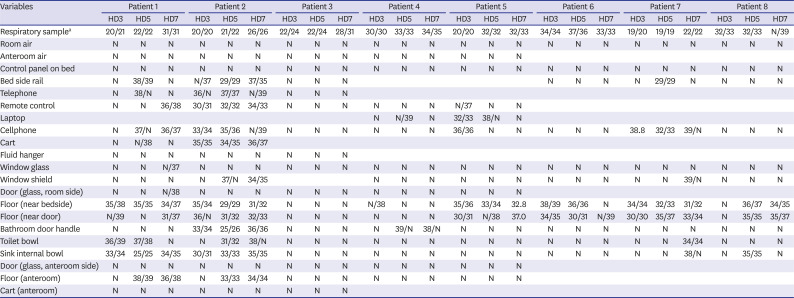
|
Variables |
Patient 1 |
Patient 2 |
Patient 3 |
Patient 4 |
Patient 5 |
Patient 6 |
Patient 7 |
Patient 8 |
|
HD3 |
HD5 |
HD7 |
HD3 |
HD5 |
HD7 |
HD3 |
HD5 |
HD7 |
HD3 |
HD5 |
HD7 |
HD3 |
HD5 |
HD7 |
HD3 |
HD5 |
HD7 |
HD3 |
HD5 |
HD7 |
HD3 |
HD5 |
HD7 |
|
Respiratory samplea
|
20/21 |
22/22 |
31/31 |
20/20 |
21/22 |
26/26 |
22/24 |
22/24 |
28/31 |
30/30 |
33/33 |
34/35 |
20/20 |
32/32 |
32/33 |
34/34 |
37/36 |
33/33 |
19/20 |
19/19 |
22/22 |
32/33 |
32/33 |
N/39 |
|
Room air |
N |
N |
N |
N |
N |
N |
N |
N |
N |
N |
N |
N |
N |
N |
N |
N |
N |
N |
N |
N |
N |
N |
N |
N |
|
Anteroom air |
N |
N |
N |
N |
N |
N |
N |
N |
N |
N |
N |
N |
N |
N |
N |
|
|
|
|
|
|
|
|
|
|
Control panel on bed |
N |
N |
N |
N |
N |
N |
N |
N |
N |
N |
N |
N |
N |
N |
N |
N |
N |
N |
N |
N |
N |
N |
N |
N |
|
Bed side rail |
N |
38/39 |
N |
N/37 |
29/29 |
37/35 |
N |
N |
N |
|
|
|
|
|
|
N |
N |
N |
N |
29/29 |
N |
N |
N |
N |
|
Telephone |
N |
38/N |
N |
36/N |
37/37 |
N/39 |
N |
N |
N |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Remote control |
N |
N |
36/38 |
30/31 |
32/32 |
34/33 |
N |
N |
N |
N |
N |
N |
N/37 |
N |
N |
|
|
|
|
|
|
|
|
|
|
Laptop |
|
|
|
|
|
|
|
|
|
N |
N/39 |
N |
32/33 |
38/N |
N |
|
|
|
|
|
|
|
|
|
|
Cellphone |
N |
37/N |
36/37 |
33/34 |
35/36 |
N/39 |
N |
N |
N |
N |
N |
N |
36/36 |
N |
N |
N |
N |
N |
38.8 |
32/33 |
39/N |
N |
N |
N |
|
Cart |
N |
N/38 |
N |
35/35 |
34/35 |
36/37 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Fluid hanger |
N |
N |
N |
N |
N |
N |
N |
N |
N |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Window glass |
N |
N |
N/37 |
N |
N |
N |
N |
N |
N |
N |
N |
N |
N |
N |
N |
N |
N |
N |
N |
N |
N |
N |
N |
N |
|
Window shield |
N |
N |
N |
N |
37/N |
34/35 |
|
|
|
N |
N |
N |
N |
N |
N |
N |
N |
N |
N |
N |
39/N |
N |
N |
N |
|
Door (glass, room side) |
N |
N |
N/38 |
N |
N |
N |
N |
N |
N |
N |
N |
N |
N |
N |
N |
|
|
|
|
|
|
|
|
|
|
Floor (near bedside) |
35/38 |
35/35 |
34/37 |
35/34 |
29/29 |
31/32 |
N |
N |
N |
N/38 |
N |
N |
35/36 |
33/34 |
32.8 |
38/39 |
36/36 |
N |
34/34 |
32/33 |
31/32 |
N |
36/37 |
34/35 |
|
Floor (near door) |
N/39 |
N |
31/37 |
36/N |
31/32 |
32/33 |
N |
N |
N |
N |
N |
N |
30/31 |
N/38 |
37.0 |
34/35 |
30/31 |
N/39 |
30/30 |
35/37 |
33/34 |
N |
35/35 |
35/37 |
|
Bathroom door handle |
N |
N |
N |
33/34 |
25/26 |
36/36 |
N |
N |
N |
N |
39/N |
38/N |
N |
N |
N |
N |
N |
N |
N |
N |
N |
N |
N |
N |
|
Toilet bowl |
36/39 |
37/38 |
N |
N |
31/32 |
38/N |
N |
N |
N |
N |
N |
N |
N |
N |
N |
N |
N |
N |
N |
N |
34/34 |
N |
N |
N |
|
Sink internal bowl |
33/34 |
25/25 |
34/35 |
30/31 |
33/33 |
35/35 |
N |
N |
N |
N |
N |
N |
N |
N |
N |
N |
N |
N |
N |
N |
38/N |
N |
35/35 |
N |
|
Door (glass, anteroom side) |
N |
N |
N |
N |
N |
N |
N |
N |
N |
N |
N |
N |
N |
N |
N |
|
|
|
|
|
|
|
|
|
|
Floor (anteroom) |
N |
38/39 |
36/38 |
N |
33/33 |
34/34 |
N |
N |
N |
N |
N |
N |
N |
N |
N |
|
|
|
|
|
|
|
|
|
|
Cart (anteroom) |
N |
N |
N |
N |
N |
N |
N |
N |
N |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|

Go to :

DISCUSSION
Appropriate respiratory precautions for COVID-19 are still being established, and airborne precautions have been recommended by some.
89 However, shortages in personal protective equipment, medical staff, and hospitals equipped with AIIR are of major concern during a pandemic. Implementing airborne precautions for all patients may not be an option in some regions. This study of environmental contamination in four different hospital settings may aid the implementation of control measures for patients with mild COVID-19 infection in whom aerosol-generating procedures have not been performed. The possibility of airborne transmission of SARS-CoV-2 has been demonstrated in vitro.
6 In addition, there have been several reports of air samples testing positive for SARS-CoV-2 RNA. However, those findings are difficult to incorporate into clinical practice because those studies had small sample sizes, lacked information on patient characteristics, and were conducted in Wuhan, where COVID-19 has a higher reproductive number than in other countries. Previous studies have reported air samples negative for SARS-CoV-2 RNA.
1011 A recent publication from Hong Kong also suggested that aerosol transmission is not a route of transmission from patients in whom aerosol-generating procedures have not been performed.
12 Our data are consistent with that study in that remote airborne transmission (≥ 2 m) was found to be uncommon in patients with mild COVID-19.
Although viral RNA does not necessarily indicate an infectious virus, widespread surface contamination of SARS-CoV-2 RNA was observed in the patients' rooms. This is consistent with previous case studies.
41011 Frequently touched surfaces tended to have higher percentage of positive SARS-CoV-2 RNA PCR, and extensive environmental contamination was observed for 7 days after admission. This was true even in the rooms of patients with only upper respiratory symptoms (patients 1 and 4). The extent of environmental contamination in our study could be attributable to contamination via direct touching of patients and/or healthcare workers after contact with infected respiratory fluids. However, in this study, SARS-CoV-2 RNA was not detected in a room routinely cleaned by disinfectant wipes. This demonstrates the importance of environmental cleaning in reducing exposure to SARS-CoV-2. However, SARS-CoV-2 RNA was detected in a room sprayed with disinfectant, suggesting that disinfectant sprays may not be effective in reducing exposure to SARS-CoV-2.
This study has several limitations. First, the number of patients was small, rendering it difficult to establish statistical significance. Studies with larger samples and more extensive statistical analyses are needed. Our study also excluded patients who had undergone aerosol-generating procedures, which have the potential to increase airborne transmission. Further studies are needed to evaluate the role of aerosols in these cases.
In conclusion, our data suggest that remote airborne transmission (> 2 m) from COVID-19 patients is uncommon when aerosol-generating procedures have not been performed. Widespread surface contamination was observed in all rooms except one that was routinely cleaned with disinfectant wipes.
Go to :

ACKNOWLEDGMENTS
We express our gratitude to the car racer Soo Woong Hwang (H-motorsports, Gwangju) who transported the specimens and instruments necessary for specimen collection between Daegu and Gwangju.
Go to :

Notes
Go to :

References
2. Liu Y, Gayle AA, Wilder-Smith A, Rocklöv J. The reproductive number of COVID-19 is higher compared to SARS coronavirus. J Travel Med. 2020; 27(2):taaa021. PMID:
32052846.

4. Chia PY, Coleman KK, Tan YK, Ong SWX, Gum M, Lau SK, et al. Detection of air and surface contamination by SARS-CoV-2 in hospital rooms of infected patients. Nat Commun. 2020; 11(1):2800. PMID:
32472043.

5. Guo ZD, Wang ZY, Zhang SF, Li X, Li L, Li C, et al. Aerosol and surface distribution of severe acute respiratory syndrome coronavirus 2 in hospital wards, Wuhan, China, 2020. Emerg Infect Dis. 2020; 26(7):1583–1591. PMID:
32275497.

6. van Doremalen N, Bushmaker T, Morris DH, Holbrook MG, Gamble A, Williamson BN, et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med. 2020; 382(16):1564–1567. PMID:
32182409.

7. Liu Y, Ning Z, Chen Y, Guo M, Liu Y, Gali NK, et al. Aerodynamic analysis of SARS-CoV-2 in two Wuhan hospitals. Nature. 2020; 582(7813):557–560. PMID:
32340022.

8. Bahl P, Doolan C, de Silva C, Chughtai AA, Bourouiba L, MacIntyre CR. Airborne or droplet precautions for health workers treating COVID-19? J Infect Dis. 2020.
9. Setti L, Passarini F, De Gennaro G, Barbieri P, Perrone MG, Borelli M, et al. Airborne transmission route of COVID-19: why 2 meters/6 feet of inter-personal distance could not be enough. Int J Environ Res Public Health. 2020; 17(8):2932.

10. Ong SWX, Tan YK, Chia PY, Lee TH, Ng OT, Wong MSY, et al. Air, surface environmental, and personal protective equipment contamination by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) from a symptomatic patient. JAMA. 2020; 323(16):1610–1612. PMID:
32129805.

11. Yung CF, Kam KQ, Wong MSY, Maiwald M, Tan YK, Tan BH, et al. Environment and personal protective equipment tests for SARS-CoV-2 in the isolation room of an infant with infection. Ann Intern Med. 2020; 173(3):240–242. PMID:
32236490.

12. Cheng VC, Wong SC, Chan VW, So SY, Chen JH, Yip CC, et al. Air and environmental sampling for SARS-CoV-2 around hospitalized patients with coronavirus disease 2019 (COVID-19). Infect Control Hosp Epidemiol. 2020.

Go to :








 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download