1) Initial model of radiation cancer therapy devices
Current cancer therapy includes radiotherapy using an X-ray beam. Treating cancer using a medical LINAC has become a main trend in cancer therapy. Clinical LINAC models currently used commercially in clinical practice have evolved continuously through many trials and errors. Until 1950, external beam radiotherapy has been performed mainly with X-rays generated at a maximum of 300 kV. In the1950s and 1960s, the existing kV machines were replaced gradually with high energy machines as the development of high energy photon-emitting accelerators and Co-60 therapy machines became highly popular. Nevertheless, kV machines with low energy beams did not fade away until recently and have existed for the treatment of special sites.
In this chapter, we review X-ray generators that were the starting point of high energy machine development and were used for the first radiotherapy procedures. They predicted the kind of machines that would be developed in the future. The Radiotherapeutic Research Unit of the Medical Research Council at Hammersmith Hospital, London, installed the first clinically usable high energy LINAC. It was used February 1953 for physics experiments and other tests. Patient treatment was attempted on September 7 the same year but it was not used for patient treatment exclusively [
15]. The Granz Ray Therapy device, which generated X-rays of less than 20 kV, was the first machine to be used for treatment before high energy X-ray generating LINAC devices. Contact Therapy generated X-rays in the range of 40–50 kV and it was used to treat patients with contact close to the treatment lesion. This therapy was suited for superficial tumors because the maximum absorbed dose was near the skin. Superficial Therapy machines were developed next. They generated X-rays in the range of 50–150 kV and were applied for radiotherapy. Orthovoltage Therapy and Deep Therapy that generated energies of 150–500 kV were developed and utilized for cancer treatment. This system had a source-surface distance (SSD) of about 50 cm and used cones to collimate the beam to the desired size. It still had a maximum dose near the skin but 90% of the dose occurred at a depth of about 2 cm below the skin, which facilitated treating the tumor deeper than with Superficial Therapy.
Fig. 2a shows graphs of the percentage depth dose for Granz to Orthovoltage with different beam qualities and maximum doses at the skin surface, while Cobalt-60 (Co-60) had its maximum dose at some distance below the skin and much less skin reaction than with the kV machines. The device (
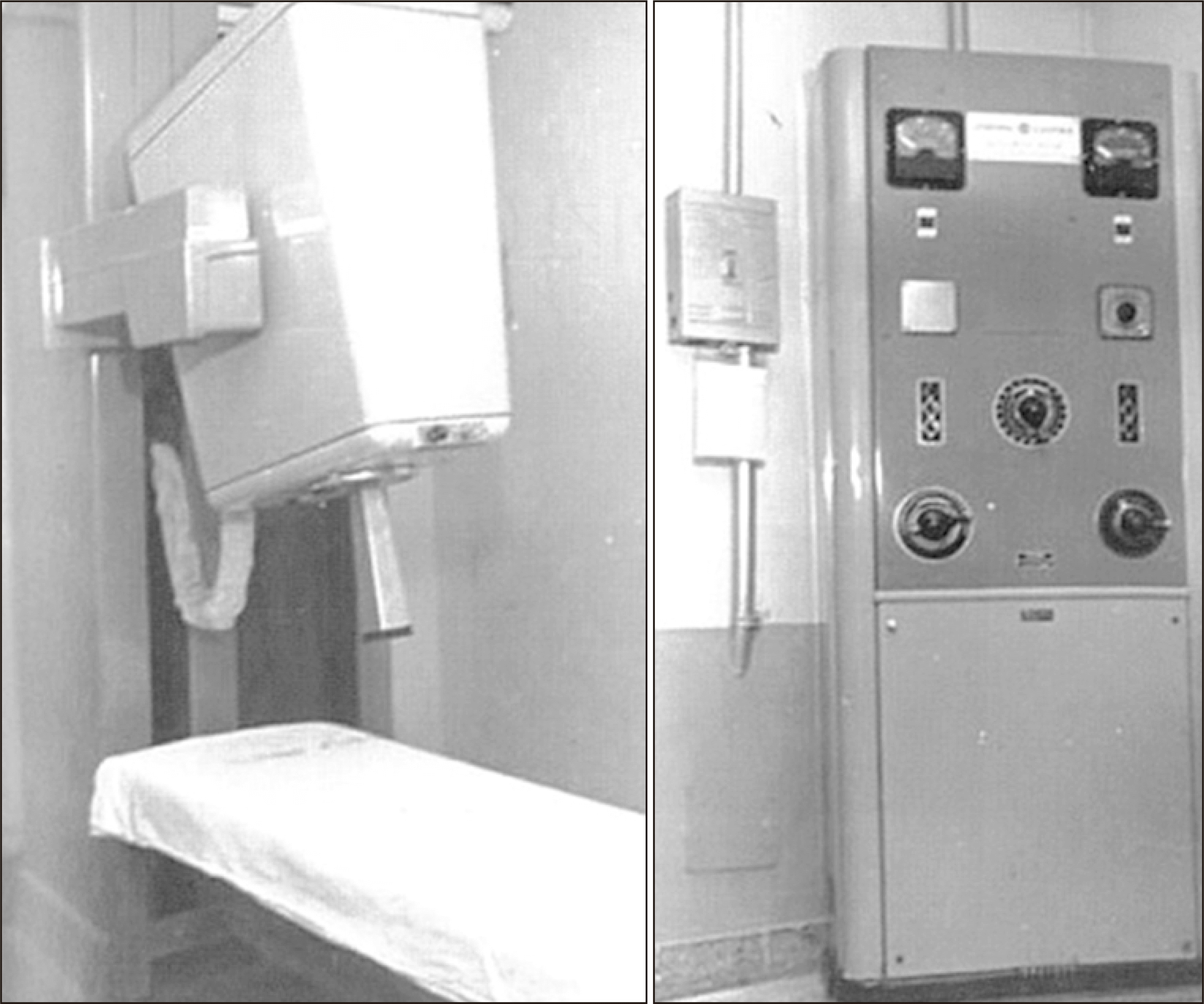
Fig. 2b) is a superficial therapy machine equipped with a cone.
Fig. 2c shows a superficial skin cancer that was treated with this type of machine.
 | Fig. 2The developmental process of kV therapy machines from Granz to Orthovoltage. (a) The graph shows percentage depth doses (PDDs) of kV X-ray therapy machines and Co-60 machines with the maximum dose at a distance below the skin. (b) The machine in picture is a superficial therapy machine equipped with a cone, and (c) the picture demonstrates that superficial skin cancer can be treated using this type of machine. 
|
However, there have been problems when the skin was exposed to large radiation doses to treat deep-seated tumors. From the 1950s to the 1960s, many efforts were made to develop a device capable of generating high energy photon beams. Using a step just before high energy, Supervoltage Therapy was developed that could generate up to 1,000 kV. The Van De Graff Generator without Co-60 teletherapy was the first to produce MV high energy. This system was an electrostatic accelerator designed to accelerate charged particles. This machine accelerated electrons to produce high energy X-ray beams of up to 2 MV in radiation therapy. This machine is not commercially produced anymore because Co-60 teletherapy machines and medical LINACs with better technical and physical advantages came to be used.
2) Development of hospital-dedicated high energy LINACs
The need for high-energy radiotherapy machines was recognized from the beginning of radiotherapy, but technical shortages hampered development. In 1953, Metropolitan-Vickers contributed to the advent of LINACs. He developed and produced a LINAC device that accelerated charged particles, such as electrons, to high energies through linear a vacuum tube using high-frequency electromagnetic waves. This LINAC produced 8 MV X-rays using 3 meter acceleration or corrugated waveguides, was used to treat patients, and was the only LINAC machine at that time [
16]. This was immediately followed by the additional installation of two LINACs using 4 MV.
One of them was installed at Newcastle by Mullard Equipment Ltd, a division of Philips, and the other was installed at Manchester Christie Hospital by Met-Vic. In addition, Met-vic supplied two more in 1955. They were installed at Western General Hospital, Edinburgh, and Mount Vernon Hospital, Middlesex [
17]. In addition, a Stanford University research group in California, USA installed a 6 MV LINAC at Stanford University in the Department of Radiology in 1954. They treated the first patient in 1956. At that time, seven LINACs were installed through the world. The 15 MV machines were installed at St. Bartholomew’s Hospital and a few medical centers in Chicago. At that time, these machines were said to be installed or under installation, but it was not clear whether they had been applied in clinical practices [
17,
18].
Three Met-Vic systems were operating in Australia from 1956 to 1957 and, not long after that, they were installed in New Zealand as well. Until 1962, the Mullard Company installed LINAC systems in Australia, Japan, and Russia. Varian Company installed the first prototype of a 6 MV isocenter LINAC at University of California, Los Angeles Medical Center. A Mevatron was installed in France in 1965 [
17]. Since then, high energy LINACs have been evolving. The development of high energy radiotherapy machines has a deep connection with that of electron beam acceleration modes.
3) Historical origin of a high energy LINAC
In 1928, the mode of electron acceleration, proposed by Ising, a Swedish physicist, and developed by Wideroe, a Norwegian physicist, connected a series of linear tubes that allowed electrons to gain energy whenever they went through the gaps (
Fig. 3). This principle had a limitation for clinical use in that the length of the accelerator tube increased with electron speed when the frequency was kept constant.
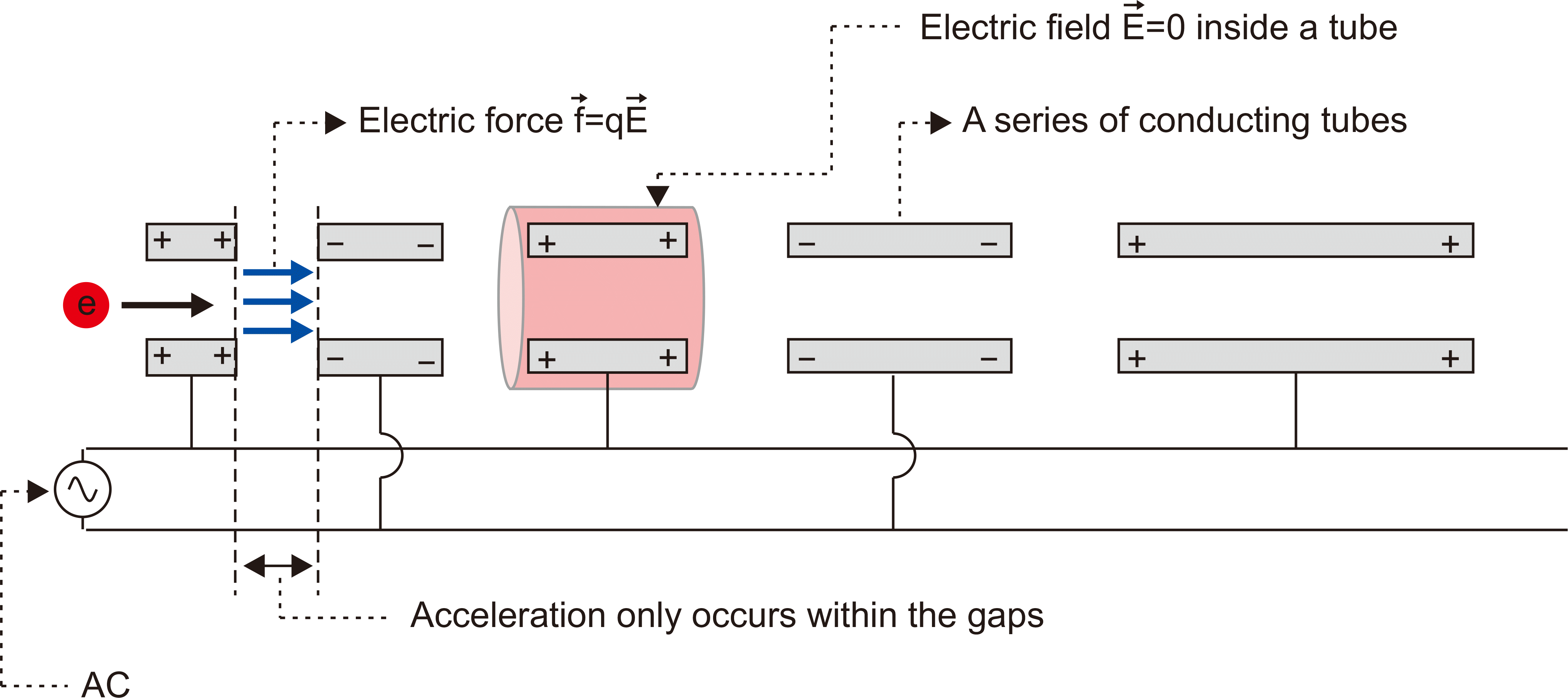 | Fig. 3Initial design of the electron acceleration mode. Electrons were drifted through a series of flight tubes connected to an alternating voltage supply (AC) and accelerated only as they passed through the gaps between the drift tubes. 
|
In the mid-1930s, Hansen from Stanford, CA, USA, developed an electron accelerating machine called Rhumbatron. This device repeatedly passed electrons through a resonant microwave cavity to increase energy when passing each section [
19]. However, the electric power level at that time from the microwave generator was inappropriate for clinical applications. Through World War II, war radar devices in the military required a microwave generator with MW output, and two tubes with this type of performance were developed. In 1939, Boot and Randall [
20] (England) designed the Magnetron, and in 1939, the Varian brothers (USA) developed the Klystron [
21]. The basic difference between these two is the Magnetron is a self-generator that generates frequencies in response to DC input, while the Klystron is, in principle, an amplifier that amplifies the low power microwave input. These war radar systems were operated at a frequency of 3 GHz, which corresponds to a wavelength of 10 cm in free space, and they were well suited for the development of electron accelerators. Since 1945, the development of LINACs using microwaves has been done independently in the United States and the United Kingdom.
For the first time, the LINAC models were developed by adopting a traveling wave mode of acceleration modes. The LINAC, which adopted the first traveling wave mode for clinical use, was installed at Hammersmith with an accelerator tube length of about 3 m and a 2 MW magnetron. The beam traveling in a horizontal direction was bent by 90 degrees to a target. It had a field size of maximum 20 cm and dose rate of around 150 rad/min. The Varian Company, USA, at first adopted the traveling wave mode as well, but in 1962, the standing wave mode was adopted by Knapp et al. [
22]. The main difference in this design was it produced an improved shunt impedance, enabling significant reduction of the accelerator size at the same energy. This design could produce 4–6 MV of energy from waveguides of 30–35 cm.
In the 1970s, LINACs were not changed dramatically in basic concept or structures, but the wedge system and the precision of the beam bending system were continuously improved. Therapy machines equipped with a general safety system were developed using the latest optimization techniques, such as a magnetron, control system, and other components. At the time of development, the advantages were not clear, but the initial therapy devices were very important in the evolution of sophisticated and modern LINACs used currently in the clinic.
Clinically available devices following physicists’ research steps are the following: basic control system, safety and support system, beam correction, patient positioning accessory, beam isotropic mounting, full rotation, vacuum pump, other vacuum component development, beam bending system, energy increase, independent collimator (MLC), electronic portal imaging device (EPID), computer control, dynamic wedge, IMRT function, and IGRT system, which has become easy to use in recent years. At the same time, various, accurate, and automated patient positioning and identification systems have been developed.
As to the development of medical LINACs, dose rates are important. Medical LINACs have experienced dramatic enhancement of dose rates since their initial development. LINAC-based radiation therapy for cancer treatment began with the first patient in 1953 in London, UK, at Hammersmith Hospital, with an 8 MV machine built by Metropolitan-Vickers and installed in 1952, which was the first dedicated medical LINAC. A short while later in 1954, a 6 MV LINAC was installed at Stanford, CA, USA, which was used for treatments in 1956 (
Fig. 4a). The first Stanford LINAC device produced output of 65 rad/min at the maximum of the transition curve in water. Conventional medical LINACs use fixed dose rates of, for example, 200 MU/min (=2 Gy/min) and 300 MU/min (=3 Gy/min) for 6 MV and 10 MV, respectively, at the depth of a maximum dose in a water phantom on the central axis beam when irradiated with a 10×10 cm
2 field at a SSD of 100.0 cm. The latest LINAC can produce a dose rate of up to 20 Gy/min. Maxim et al. [
23] are developing pluridirectional high-energy agile scanning electronic radiotherapy (PHASER) as a platform for clinical translation of FLASH (mentioned briefly in Chapter 3) cancer radiotherapy (
Fig. 4b), which requires dose rates exceeding ~50 Gy/s. Such a rapid rise of dose rates reflects one aspect of the future trends of clinical LINACs.
 | Fig. 4From the initial medical LINAC to a future design under development. Dr. Kaplan and physicist Ginzton developed the first 6 MV medical linear accelerator (a) in the Western hemisphere in 1953, which was used to treat a 2 year-old boy with retinoblastoma in 1956 (Stanford Report, April 18, 2017). (b) Maxim et al. are developing pluridirectional high-energy agile scanning electronic radiotherapy (PHASER), which aims to be a platform for clinical translation of FLASH cancer radiotherapy [ 23]. 
|
4) Development status of LINACs in Korea
The radiation cancer therapy devices installed and used for cancer treatment in about 100 medical institutions in Korea are all imported from abroad. Many research groups in Korea have been participating in the government-led development of medical LINACs from 10 years ago to achieve technological independence.
In 2012, Dr. Young-Hoon Ji’s research team at the Korea Atomic Energy Research Institute attempted to develop a Korean LINAC supported by the Ministry of Science and ICT, and Future Planning with the aim of providing them to developing countries and Korea. They did not reach the stage of application due to a lack of research funding, but succeeded in the development of a general purpose MLC and treatment planning system. Supported by the Ministry of Knowledge Economy in 2012, professor Chae Jong-seo at Sungkyunkwan University, aimed to develop a dual-head LINAC designed to reduce 30% of the treatment time through joint participation from the Korea Atomic Energy Research Institute, Yonsei University, Pusan National University, Kyungpook National University, and Korean companies.
At the same time, the team of Dr. Kyung-Min Jeong at the Korea Atomic Energy Research Institute conducted the development of a LINAC radiotherapy machine equipped with high-performance radiation for therapy and an imaging system supported by the Ministry of Science and ICT. As a segment of leading industry and promoting industry in metropolitan economic blocks in 2012, Taesung Precision Co., Ltd led the development of a high-powered X-ray and electron beam LINAC equipped with a small accelerator using an S-band for clinical applications and bio-industry in cooperation with the Atomic Energy Medical Center in the southeast of Korea. In Korea, the government-led development of LINACs has been attempted many times. Such efforts were not put to practical use because of the lack of lasting support from the government and poor participation from companies, but constant interest from related professionals has been making continuous accumulation of related technologies.






 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download