Abstract
Neurolymphomatosis (NL) is characterized by the infiltration of malignant lymphoma cells into peripheral nerves, nerve roots, plexuses, or cranial nerves. This is a very rare complication of mantle-cell lymphoma. Diagnosing NL is made difficult by cerebrospinal fluid cytology and bone-marrow biopsy results often being negative. NL can appear as the only sign of recurrence in a patient with a previous diagnosis of lymphoma. Here we present two cases of NL in patients with mantle-cell lymphoma diagnosed by positron emission tomography with deoxy-fluoro-D-glucose integrated with computed tomography.
Neurolymphomatosis (NL) is an uncommon disease characterized by the infiltration of malignant lymphoma cells into peripheral nerves, nerve roots, plexuses, or cranial nerves. NL is frequently associated with B-cell non-Hodgkin’s lymphoma (NHL), and especially diffuse large B-cell lymphoma [1]. Mantle-cell lymphoma (MCL) is a rare subtype of NHL that derives from B-cells. NL associated with MCL is extremely rare [2].
NL can present with various clinical features, including cranial neuropathy, mononeuropathy, multiple mononeuropathy, polyneuropathy, plexopathy, and polyradiculopathy. Cauda equine syndrome is a rare manifestation of NL that is sometimes combined with spinal cord involvement [1]. Diagnosing NL is challenging because of the variability in its clinical features [3], numerous differential diagnoses [4], and frequent negative findings in cerebrospinal fluid (CSF) cytology [5]. Here we describe two cases of NL that presented as cauda equine syndrome in patients with MCL diagnosed by positron emission tomography with deoxy-fluoro-D-glucose integrated with computed tomography (FDG PET-CT).
A 71-year-old woman presented with left thigh pain and subjective weakness that had first appeared about 20 days previously. She had been diagnosed with MCL 6 months previously, and had been treated six times with bortezomib, rituximab, cyclophosphamide, and doxorubicin. She had felt coldness and tingling in both legs since receiving chemotherapy. A neurological examination revealed decreased vibration sensation symmetrically with normal motor power and symmetric hyporeflexia on the ankle. A nerve conduction study (NCS) showed early polyneuropathy. About 10 days later she experienced voiding difficulty following diplopia. A neurological examination revealed additional findings that included bilateral sixth cranial nerve palsy, mild motor weakness (Medical Research Council grade 4) of both legs, and hyporeflexia in both knees and ankles. A CSF study showed a yellowish color, leukocytosis with predominant mononuclear cells (340/mm3), and elevated total protein (566 mg/dL). No malignant cells or infectious causes including tuberculosis were observed in the CSF. A bone-marrow biopsy revealed complete remission of the previous lymphoma and no malignant cells. Leptomeningeal enhancement with bilateral thickening of trigeminal nerves was observed in brain magnetic resonance imaging (MRI) (Fig. 1A-D). Leptomeningeal enhancement of the thoracolumbosacral area was found in spine MRI (Fig. 1F), as well as diffuse enhancement of multiple spinal nerve roots (Fig. 1G). Compared to the previous study that was performed after diagnosing MCL (Fig. 1H), FDG PET-CT performed at presentation showed that the previous lesion had completely resolved and revealed a new linear area of high uptake in the lower thoracolumbar spinal cord and cauda equine (Fig. 1I). Based on a diagnosis of relapse of MCL as NL, the patient was started on ibrutinib with high-dose methotrexate. The patient expired due to pneumonia one month later.
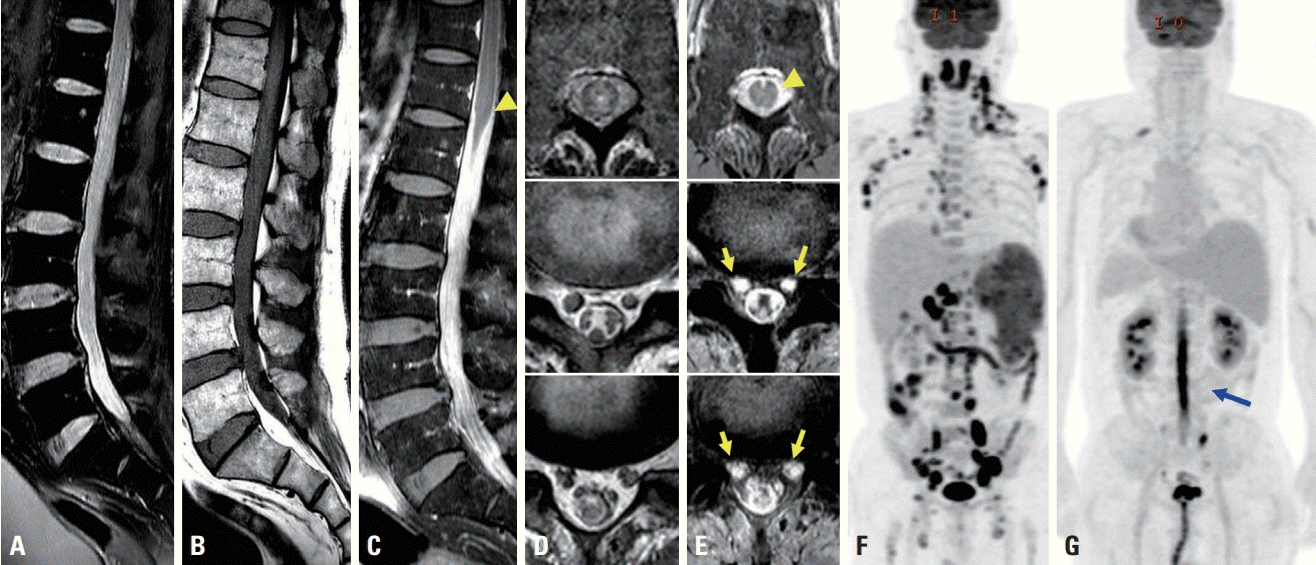
A 62-year-old man who had been diagnosed with MCL 2 months previously was referred to us due to neuropathic pain on both thighs during the third phase of chemotherapy with cyclophosphamide, vincristine, doxorubicin, and dexamethasone. He felt severe neuropathic pain on both posterior thighs, and this pain was aggravated when supine or sitting. A neurological examination revealed bilateral hypesthesia in the S1 and S2 dermatome areas and hyporeflexia on the ankle. Acute-stage radiculopathy from L5 to S2 was suspected from the NCS and electromyography. He complained of worsening neuropathic pain and difficulties in urination and defecation 1 week later. A CSF study showed a yellowish color, leukocytosis with predominant mononuclear cells (1,210/mm3), and elevated total protein (>3,000 mg/dL). There were no malignant cells or infectious causes including tuberculosis in the CSF. Spine MRI showed diffuse leptomeningeal enhancement from L1 to S1 (Fig. 2C) with diffuse enlargement of spinal nerves (Fig. 2D, E). FDG PET-CT showed a linear hypermetabolic lesion in the spinal cord from T11 down to the cauda equine (Fig. 2G) and complete remission of previous multiple lymph-node lesions (Fig. 2F). He was diagnosed with NL presenting as cauda equine syndrome and treated with a methylprednisolone pulse and rituximab. However, the patient did not improve, and expired 2 days later.
MCL accounts for 5-10% of lymphomas. It is frequently diagnosed in an advanced disease state with generalized lymphadenopathy and splenomegaly. The disease has an adverse clinical course characterized by a poor response to chemotherapy and a median survival period of 3-5 years [6]. Central nervous system involvement reportedly occurs in 4-23% of patients and leads to a worse prognosis, with a median survival period of 3 months [7]. NL is a rare condition that affects peripheral nerves, roots, or cranial nerves, and typically presents as a neuropathy. NL of MCL is extremely rare, with only a few case series having been reported [2].
NL frequently occurs in patients with known lymphoma, but less frequently as a primary manifestation of lymphoma. Previous studies found that 74-79% of patients were diagnosed with NL after known lymphoma recurrence or progression, with 63-67% of cases of NL being the only site of lymphoma at the time of diagnosis [1,3]. Prior studies have shown that nerves may act as a “safe lymphoma haven” due to chemotherapeutic agents not being able to cross the blood–nerve barrier. Therefore, when it occurs, NL is often the only site of lymphoma involvement, independent of any history of prior treatment [3]. The first of the present cases was diagnosed with NL as a recurrence of lymphoma, while the second case was diagnosed with NL as a progression of previous lymphoma. The spinal cord and cauda equine were the only FDG uptake sites with remission of previous lesions in FDG PET-CT in these cases. Clinicians should therefore be aware of the FDG PET-CT findings of NL, with the possibility that FDG uptake is isolated only in nerves regardless of the lymphoma status.
Diagnosing NL is very difficult because the sensitivity of diagnostic tests varies between cases and positive findings in these tests are not specific for NL. MRI is the useful imaging modality for diagnosing NL. Typical MRI findings in NL are spinal root or nerve enlargement and/or enhancement, but these are not specific for NL since they can also be observed in inflammatory radiculopathies or nerve-sheath tumors [8]. CSF analysis typically shows elevated protein, low glucose, and elevated cell counts [2]. Only a minority of NL cases have malignant cells in CSF [5]. Abnormal CSF findings also do not indicate whether there is any nerve involvement, and so a biopsy of an involved nerve may be useful [2]. The sensitivities of MRI, nerve biopsy, and CSF analysis have been reported to be 40-77%, 80-88%, and 21-40% respectively [3,9,10]. The sensitivity of FDG PET-CT has been reported at 84-100%, which is comparable to that of nerve biopsy [1,3,10]. An elevated rate of glycolysis–as occurs in infection or inflammation– could cause FDG uptake, thus producing false-positive FDG PET-CT findings in some cases. However, this could be the most-sensitive method for diagnosing NL, especially in patients with suspected recurrence or progression of NHL.
The present two cases illustrate the clinical feature of NL as an isolated manifestation of lymphoma and the usefulness of FDG PET-CT for diagnosing NL. Although the prognosis of these cases was poor, the rapid diagnosis and treatment of NL could increase the median survival period of such patients. Clinicians should pay attention to FDG PET-CT findings in any patient with the clinical symptoms of neuropathy, especially if they have previously been diagnosed with lymphoma.
REFERENCES
1. Davidson T, Kedmi M, Avigdor A, Komisar O, Chikman B, Lidar M, et al. FDG PET-CT evaluation in neurolymphomatosis: imaging characteristics and clinical outcomes. Leuk Lymphoma. 2018; 59:348–356.

2. Pham M, Awad M. Lymphoma relapse presenting as neurolymphomatosis. Asian J Neurosurg. 2016; 11:73.

3. DeVries AH, Howe BM, Spinner RJ, Broski SM. B-cell peripheral neurolymphomatosis: MRI and (18)F-FDG PET/CT imaging characteristics. Skeletal Radiol. 2019; 48:1043–1050.

4. Vargas TC, Thomas RL, Erickson JC. Leptomeningeal enhancement in a patient with progressive cranial neuropathies and lumbosacral radiculopathies. JAMA Neurol. 2016; 73:345–346.

5. Lin M, Kilanowska J, Taper J, Chu J. Neurolymphomatosis–diagnosis and assessment of treatment response by FDG PET-CT. Hematol Oncol. 2008; 26:43–45.

6. Ferrer A, Bosch F, Villamor N, Rozman M, Graus F, Gutiérrez G, et al. Central nervous system involvement in mantle cell lymphoma. Ann Oncol. 2008; 19:135–141.

7. Faivre G, Lagarde J, Choquet S, Maillart E, Lubetzki C. CNS involvement at diagnosis in mantle cell lymphoma with atypical MRI features. J Neurol. 2014; 261:1018–1020.

8. Baehring JM, Damek D, Martin EC, Betensky RA, Hochberg FH. Neurolymphomatosis. Neuro Oncol. 2003; 5:104–115.

Fig. 1.
Brain magnetic resonance imaging (MRI) (A-D), spine MRI (E-G), and positron emission tomography with deoxy-fluoro-D-glucose integrated with computed tomography (FDG PET-CT) for diagnosing mantle-cell lymphoma (H) and neurolymphomatosis (I). There were no abnormal signal intensities in diffusion weighted image (A) and T2-weighted (B) images. Diffuse thickening and bilateral enhancement (arrowheads) of the trigeminal nerve were observed in T1-weighted gadolinium-enhanced images (C, D). Diffuse leptomeningeal enhancement (arrowheads) was observed on a T1-weighted gadolinium-enhanced image (F) compared to a nonenhanced T1-weighted image (E). Multiple spinal nerve enhancements (arrows) were observed in a T1-weighted gadolinium-enhanced image (G). Multiple regions exhibiting FDG uptake were observed in the lymph nodes and bone marrow in initial FDG PET-CT (H). Complete remission of the previous lesion with new linear area (blue arrow) of high uptake in the lower spinal cord and cauda equine were observed in subsequent FDG PET-CT (I).
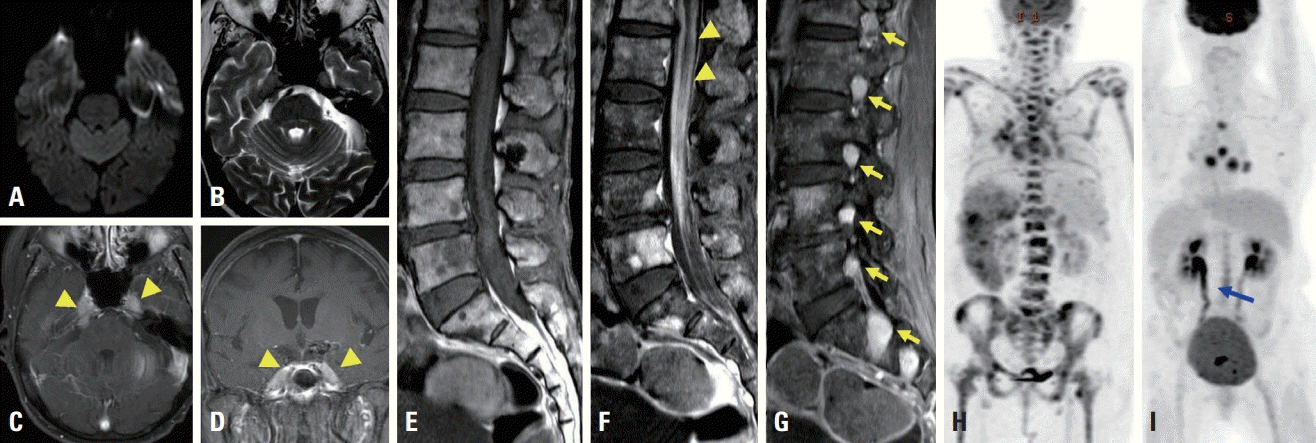
Fig. 2.
Spine magnetic resonance imaging (A-E) and positron emission tomography with deoxy-fluoro-D-glucose integrated with computed tomography for diagnosing mantle-cell lymphoma (F) and neurolymphomatosis (G). Enlargement of the spinal nerves was observed in T2-weighted (A) and T1-weighted (B) image. Diffuse leptomeningeal enhancement (arrowheads) was observed in T1-weighted gadolinium-enhanced images (C, E). Multiple spinal nerve roots were enhanced (arrows) after gadolinium injection (E) compared to a T2-weighted axial image (D). Multiple lymph nodes showed hypermetabolism in initial FDG PET-CT (F). Thoracolumbar spinal cord hypermetabolism (blue arrow) with improvement of the previous lesion was evident in subsequent FDG PET-CT (G).




 Citation
Citation Print
Print



 XML Download
XML Download