Abstract
Low-carbohydrate diets have become increasingly popular in both media and clinical research settings. Although they may improve some metabolic markers, their effects on arterial function remain unclear. Endothelial dysfunction is the well-established response to cardiovascular risk factors and a pivotal feature that precedes atherosclerotic diseases. It has been demonstrated that a high carbohydrate-induced hyperglycemia and subsequent oxidative stress acutely worsen the efficacy of the endothelial vasodilatory system. Thus, in theory, a carbohydrate restricted diet may preserve the integrity of the arterial system. This review attempts to provide insight on whether low-carbohydrate diets have a favorable or detrimental impact on vascular function, or it is perhaps the quality of carbohydrate that should direct dietary recommendations. Research to date suggests that diets low in carbohydrate amount may negatively impact vascular endothelial function. Conversely, it appears that maintaining recommended carbohydrate intake with utilization of low glycemic index foods generates a more favorable vascular profile. Understanding these relationships will aid in deciphering the diverging role of modulating quantity and quality of carbohydrates on cardiovascular risk.
According to current dietary recommendations, 45-60% of daily energy intake should be sourced from carbohydrates [1]. Diets that restrict carbohydrate consumption have been endorsed as a healthier alternative and are a popular strategy for weight loss [2]. While they may improve some metabolic markers, the support for low-carbohydrate (low-CHO) diets is clouded by a recent meta-analysis that suggests that these diets do not appear to be protective from cardiovascular (CV) incidence and death [3]. Given that the vascular endothelial function can provide early prognostic value of CV events and is one of the key regulators of atherosclerotic processes, the net impact of dietary carbohydrate manipulation on endothelial function should be further understood.
Carbohydrate restriction is largely driven by the notion of sugar 'toxicity', or the deleterious effects of carbohydrate-induced hyperglycemia. However, the relationship between carbohydrate and hyperglycemia is also influenced by carbohydrate quality rather than simply quantity, which has been conceptualized as glycemic index (GI). An argument is emerging on whether improving carbohydrate quality (low-GI diets) as opposed to reducing carbohydrate load (low-CHO diets) is a more valuable strategy for vascular protection when considering a comprehensive assessment of cardiovascular disease (CVD) risk. The purpose of this review is to examine available evidence and define the relationship between quantity or quality of carbohydrate intake and the impact on the vascular endothelial function. Studies evaluating endothelial function in humans by brachial ultrasonography using flow mediated vasodilatation were mainly considered.
A healthy endothelium is essential for maintaining vascular health through regulation of key processes, including blood flow via nitric oxide production, coagulation, and smooth muscle cell proliferation [4]. When the vasculature is challenged by proathersclerotic stimuli, including hyperglycemia, the endothelium changes to a pathogenic phenotype, ultimately damaging the vessel wall. Accordingly, endothelial dysfunction is thought to be an initial step in the setting of atherosclerosis and CVD and is an independent predictor of CV events in both healthy individuals and those at risk for CVD [5]. Dysfunction of the vascular endothelium is typically defined as a reduced response to vasodilatory stimuli. It is often evaluated by an ultrasound-based technique of the brachial artery and expressed as a percentage of artery dilatation following a temporary vessel occlusion (flow-mediated dilation, FMD) [6,7]. Each unit decrease in FMD, a marker of endothelial function, has been linked to a 13% increased risk of CV events [5].
Hyperglycemia is postulated to induce a cascade of events that are detrimental to endothelial function. Studies that have utilized exposure to acute glucose elevations have demonstrated a notable impact on production of reactive oxygen species as well as increased adhesion molecule expression, vascular permeability and secretion of plasminogen activator inhibitor-1 (PAI-1) [8]. Glucose can enter endothelial and vascular smooth muscle cells via glucose transporter 1 (GLUT-1). However, hyperglycemic states detrimentally affect the equilibrium of intracellular proteins, where glucose moieties bind to amine groups on proteins, leading to formation of advanced glycation end products (AGEs) [9]. AGEs are glycated proteins or lipids within the vessel wall that form crosslinks within the extracellular proteins and upregulate transcription factors which modify the structure and function of the vasculature. Via another pathway, high glucose influx has been implied in accumulation of diacylglycerol and activation of diacylglycerol-protein kinase C (PKC) cascade, where PKC-mediated phosphorylation impairs anti-inflammatory insulin action [10]. Increase in PKC leads to elevations in the nuclear factor kB, tumor necrosis factor alpha and PAI-1, all strongly implicated in promoting vascular pathogenesis. Moreover, a high flux of glucose can be metabolized in certain cells to sorbitol and fructose via a sorbitol-aldose reductase pathway [11]. These molecules deplete antioxidant protection and elevate circulating cytokines [12]. Thus, in the endothelium, increases in pro-inflammatory mediators through these channels set in motion a feed forward cycle that further leads to more inflammation, foam cell formation, thrombosis, and proliferation to concomitantly affect endothelial integrity [6,13]. More recently, studies have also suggested that glucose elevations initiate epigenetic changes in gene promoters that may lead to continuous inflammation following acute exposure [14]. Hence, postprandial acceleration of oxidative stress and inflammation through hyperglycemia has a profound effect on vascular function.
The endothelial impact of oral carbohydrate challenges has been investigated in several settings. Ceriello et al. [15] was the first to show a reduction in postprandial endogenous antioxidant levels and an increase in a marker of endothelial damage following an oral glucose tolerance test (OGTT) in healthy individuals. A significant decrease in FMD following an OGTT was subsequently observed by independent investigators [16,17,18]. Pre-treatment with either vitamin C or a statin during an OGTT attenuated postprandial endothelial impairment following an OGTT alone [19,20], suggesting a clear oxidative-stress link. Similarly, when an effect of a high carbohydrate challenge was examined in individuals with diabetes, the results uniformly show postprandial elevations in oxidative stress markers and impaired endothelium function, often to a greater extent than that observed in healthy individuals [20,21,22]. A causative link between hyperglycemia and endothelial function may be inferred by these studies which provide consistent evidence that, in an acute setting, a high carbohydrate load will adversely affect postprandial endothelial events.
With westernization of dietary patterns, individuals are spending considerably more time in a postprandial state, identified as being a critical period for atherosclerotic plaque formation [23]. From this standpoint, it is reasonable that dietary approaches to lower postprandial glycemia may have a positive effect on endothelial function and atherosclerotic progression. Low-CHO or low-GI diets are both venues by which a lower postprandial glycemia can be achieved.
Low-CHO diets are a class of dietary patterns that source less than 45% of energy from carbohydrates. These regimens are expected to induce weight loss and improve cardio-metabolic risk factors. Within the literature, low-CHO diets are most often higher in total fat and are compared to high-CHO dietary concepts compensating for a reduced fat intake. Regardless of apparent benefits favoring low-CHO diets within the initial 6 months of intervention [24], both diets have similar outcomes on weight loss, blood pressure, and glycemic marker reductions within one year, not encompassing diabetic populations [24,25,26]. However, the most recent meta-analysis pooling outcomes of low-CHO intake from randomized clinical trials and observational studies linked CHO restriction with a 30% increased risk of mortality from all-causes, with a modest relative risk of 1.10 for CV events [3]. The mechanisms or physiological effects that underpin the positive correlation between low-CHO diet and all-cause death are not fully explained. Therefore, to determine if a macronutrient distribution limited in carbohydrates is neutral or harmful to vascular health, we need to comprehensively consider the effects of low-CHO diet vascular endothelial function, an early marker of CVD risk.
The implications of low-CHO diets on endothelial function have been investigated in a number of trials to date (Table 1). A cross-sectional study of a high Framingham risk score population illustrated that carbohydrate intake of only 15% below recommendations was associated with the poorest endothelial function profiles, independent of major CVD risk confounders [27]. However, a restriction of this magnitude did not appear to affect FMD in overweight and obese populations [28,29]. On the other hand, this population had a significantly decreased vascular reactivity when carbohydrates account for less than 5% of energy, as in an Atkins style diet, compared to carbohydrate intakes within recommended ranges [30,31]. In these latter trials, there was no difference in weight loss between the two dietary patterns. Additionally, there was no effect of the interventions on endothelium-independent dilatation, implying that the endothelium was a mediator of the diminished response. It appears that the effects on vascular endothelium function is impacted by the severity of the carbohydrate restriction and may differ in the presence of CVD risk factors such as obesity.
Recently, data from six randomized controlled trials investigating the effects of low-CHO intake for a minimum of 3 weeks on endothelial function were pooled in a meta-analysis of 210 participants [32]. The collective evidence indicated a 1.01% decrease in FMD following a low-CHO compared to a moderate-CHO intervention in overweight or healthy adults free of coronary heart disease. This is a highly noteworthy finding given that a reduction of 1% FMD has a marked effect on future CVD events [5]. While the collective evidence favors reduced FMD in the context of a low-CHO diet, it is necessary to appreciate the complexity of evaluating dietary interventions. The effects of these popular diets on vascular health may be inherently attributed to the associated decreased intake of fiber, fruit or root vegetables, and/or the increased consumption of protein dense products such as meat and dairy, that are likely relied on for satiation [33]. These factors may contribute to the adverse vascular outcomes of low-CHO diets in long-term investigations and are in line with the different associations in CVD risks seen from diets with plantbased compared to animal-based protein sources [34]. Animal based protein sources are linked to a higher intake of saturated fat which was previously believed to be detrimental to heart health, although this is now under debate. One group observed a 50% reduction in FMD following 3 weeks of increased saturated fat consumption in the context of two high-CHO diets [35]. It is implausible to draw conclusions from this one shortterm trial and limited clinical trials consider saturated fat in their dietary interventions. It is thus worth exploring these relationships further with more rigorous trial designs.
Nonetheless, the macronutrient replacement needed to maintain energy intake complicates the study methodologies and data analysis and often makes the results difficult to interpret. With fat and protein inherently substituted for carbohydrate, it can be challenging to differentiate the effect of carbohydrate restriction from the effects due to alterations in other macronutrients.
Ultimately, further trials are required to confirm the mechanisms of endothelial function impairment following regimens of carbohydrate restriction to varying degrees. Regardless, with weight loss from restricted carbohydrate intake likely irrelevant within one year, and the possible harmful effects of these diets on endothelial function, we are overdue to consider an alternative dietary modification for decreasing glycemic load and ultimately improving, or maintaining, vascular health.
While traditional advice has centered on carbohydrate counting, it is now recognized that the type of carbohydrate is also important in predicting an individual's glycemic response. Glycemic index is the quantification of the blood glucose response to a carbohydrate in comparison to a carbohydrate reference, generally white bread or glucose [36]. The GI provides a numeric classification of carbohydrate foods, measured within person, which is thought to be indicative of the quality of the carbohydrate. An increasing number of studies are demonstrating that low and high GI foods have considerably different effects on metabolism [37,38,39]. Low-GI diet plans have proven to increase β-cell insulin production in the presence of impaired glucose tolerance [37] and show benefits on glycemic control that are carried over to subsequent meals [39]. Thus, rather than lowering the carbohydrate portion of the diet, sustaining a recommended macronutrient distribution of 45-65% carbohydrate with a focus on GI may be an important consideration in dietary management which can extend to aid in the preservation of vascular function.
Albeit limited, data is emerging from observational studies that have explored the implications of varying carbohydrate sources on endothelial function. Most recently, the latest sub-analysis of the EVIDENT cohort aimed to define the association between GI and vascular function via a measure of arterial stiffness, augmentation index (AI), in a population free of CVD [40]. AI is a novel surrogate measure of vascular aging that is related to endothelial dysfunction. Even with adjustments for multiple confounders, every unit increase in GI was significantly associated with a 0.11% increase in AI, and hence elevated risk of CVD [41,42].
While this is still a novel area of investigation, these associations were corroborated by the two randomized controlled trials undertaken in this field. Lavi et al. [43] was first to examine the 2-hour postprandial effects of varying the GI of a meal on FMD in overweight/obese individuals. Despite equal carbohydrate quantities administered, a low-GI (GI = 40) fiber cereal produced a significantly higher vasodilatory response when compared to glucose (GI = 100), suggesting a differential effect on vasodilatory mechanisms. The evaluation of a longer low-GI dietary intervention on vasodilation was more recently explored, also in an obese population [44]. The impact on endothelial function of 3-month consumption of hypocaloric diets with similar macronutrient distribution and of either low- or high-GI was explored. Although both groups showed similar weight loss, FMD was increased by 2.3% following the low-GI diet, which was significantly higher than the 0.9% decrease observed from the high-GI intervention.
A recent animal study provides support to the clinical observations, which investigated the postprandial endothelial function and oxidative stress marker, an AGE precursor, of simple versus complex carbohydrates ranging in GI, in six beagle dogs [45]. The combined response to the complex carbohydrates significantly improved FMD by 1.6% and an oxidative stress marker methylglyoxal, in line with clinical findings. Although this study was underpowered to detect differences in FMD between individual carbohydrate sources, it offers some indication of the benefit of complex carbohydrates and lower GI foods in the context of vascular function. Ultimately, low-GI dietary interventions appear, to date, to be more powerful in improving endothelial function in short and medium-term settings. However, it is necessary to appreciate the limited clinical research exploring this relationship. Epidemiological data do offer some insights, demonstrating a pooled relative CVD risk of 1.19 for highest versus lowest GI categories as reported in a recent meta-analysis [46,47]. A concurrent meta-analysis by Dong et al. confirmed this relationship [48]. Nonetheless, further randomized clinical trials are needed to draw an impactful conclusion on the vascular benefits of GI-manipulated diets. A study is currently underway to assess the effect of low-GI diets on arterial damage beyond existing cohort evidence supporting the role of low-GI diets in CVD event reduction [49].
Carbohydrates are a dietary staple that can pose as strong modulators of vascular function. With a high carbohydrate load apparently detrimental to endothelium dependent processes and associations with increased CVD risk, an emphasis has been placed on decreasing dietary glycemic load. This can be achieved by either reducing the total carbohydrate intake or lowering the GI of carbohydrate foods. However, the physiological effects of these changes are likely to be different, and this review provides a clear example of diverging vascular responses when the two dietary patterns are concerned.
Available evidence indicates that carbohydrate restriction does not appear to be a viable dietary strategy in the context of their effects on early stages of atherogenesis. While low-CHO diets may have short-term weight loss and some metabolic benefits, their utilization has largely demonstrated as deleterious on endothelial function in dietary feeding trials. These observations may provide insights into recent associations of low-CHO diets with increased mortality. It appears that a U-shaped relationship is emerging where both high and low dietary carbohydrate intakes may be associated with adverse outcomes. Thus, it may be time to reconsider a guideline-recommended macronutrient distribution to remain as a more optimal approach for preserving vascular integrity.
Within current dietary macronutrient recommendations, maneuvering the quality of carbohydrate foods may be a more promising alternative in associations with vascular health. Low-GI foods appear to have vaso-protective benefits relative to their high-GI counterparts for an equal carbohydrate quantity.
However, well designed trials with fine distinction of carbohydrate quantity and quality are needed. Within this category is only one recent, well conducted study of 5 weeks which methodically compared the effects of reducing glycemic load by decreasing carbohydrate intake versus improving carbohydrate quality (i.e. decreasing GI) in the context of a DASH or OmniHeart diet plan [50]. It demonstrated that both GI and carbohydrate load pose similar advantages on certain CVD risk factors when established healthful diets are imposed in an overweight but otherwise largely healthy population. It also supports the need to assess carbohydrate manipulations on emerging and early markers of CVD risk through similar rigorous clinical designs.
While out of the scope of this review, the vascular mechanisms of varying carbohydrate classes is a topic of growing interest with the increasing popularity of low-FODMAP (fermentable oligo-dimonosaccharides and polyols) diets and recent investigations of high-fructose corn syrup. The health outcomes of fructose, in particular, are being systematically dissected through a series of meta-analyses [51,52,53,54], but the effects on FMD per se have yet to be reviewed. Limited studies with this focus, however, have suggested a neutral influence on the vascular endothelial in acute settings [55], particularly in isocaloric comparisons with different carbohydrate classes [56].
Ultimately, a focus on macrovascular endothelial function markers in assessment of CVD risk should be more widely considered, given that the endothelium is a target of multiple metabolic processes. Thus, deciphering the mechanisms that link the low-CHO diets to endothelial dysfunction and as well as longer term validation of low-GI in the context of conventional macronutrient distribution will further our understanding of the role of carbohydrates in vascular health and ultimately enhance dietary therapeutic options.
Figures and Tables
Table 1
Studies evaluating the impact of low carbohydrate interventions and low glycemic index interventions on markers of endothelial function
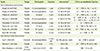
| Study | Design | Participants | Duration | Intervention* | Effect on endothelial function |
|---|---|---|---|---|---|
| Low Carbohydrate Interventions | |||||
| Keogh JB, 2007 [29] | Parallel | 13 overweight/obese | 1 year | LC (33) or HC (60) | LC ↔ FMD vs. BL; ↔ vs. HC |
| Keogh JB, 2008 [26] | Parallel | 66 overweight/obese | 8 wks | LC (4) or HC (46) | LC ↔ FMD vs. BL; ↔ vs. HC |
| Phillips SA, 2008 [57] | Parallel | 20 obese | 6 wks | LC (4) or HC (57) | LC ↓ FMD vs. BL; ↓ vs. HC |
| Buscemi S, 2009 [58] | Parallel | 20 overweight/obese | 2 mo | LC (20) or HC (55) | LC ↓ FMD vs. BL; ↓ vs. HC at 1 wk |
| LC ↔ FMD vs. BL; ↔ vs. HC at 2 mo | |||||
| Wycherley TP, 2010 [31] | Parallel | 49 overweight/obese | 1 year | LC (4) or HC (46) | LC ↓ FMD vs. HC |
| Varady KA, 2011 [30] | Parallel | 17 obese | 6 wks | LC (5) or HC (55) | LC ↓ FMD vs. BL; N/A vs. HC |
| Mohler ER, 2013 [59] | Parallel | 121 healthy | 2 years | LC (-10) or HC (55) | LC ↔ FMD vs. BL; ↔ vs. HC |
| Ruth MR, 2013 [60] | Parallel | 55 obese | 12 wks | LC (10) or HC (56) | LC ↔ FMD vs. HC |
| Low Glycemic Index Interventions | |||||
| Lavi T, 2009 [43] | Crossover | 56 overweight/obese |
4 visits (2h) |
LGI, HGI, glucose, water | LGI ↑ FMD vs. glucose |
| Buscemi S, 2013 [28] | Parallel | 40 obese | 3 mo | LGI or HGI | LGI ↑ FMD vs. BL and vs. HGI |
| Recio-Rodriguez JI, 2015 [40] | Cross-sectional | 1,553 free of CVD | N/A | LGI vs. HGI | HGI ↑ AI vs. LGI |
AI: augmentation index, CVD: cardiovascular disease, FMD: flow mediated dilation, LC: low carbohydrate, HC: high carbohydrate, HGI: high glycemic index, LGI: low glycemic index, BL: baseline, ↑: improved, ↓: impaired, ↔: unchanged.
*Carbohydrates are expressed as a percentage of daily energy intake as LC(%) or HC(%).
References
1. Macronutrients, healthful diets, and physical activity. In : Otten JJ, Hellwig JP, Meyers LD, editors. Dietary reference intakes: the essential guide to nutrient requirements. Washington, D.C.: The National Academies Press;2006. p. 70.
2. Hite AH, Berkowitz VG, Berkowitz K. Low-carbohydrate diet review: shifting the paradigm. Nutr Clin Pract. 2011; 26:300–308.
3. Noto H, Goto A, Tsujimoto T, Noda M. Low-carbohydrate diets and all-cause mortality: a systematic review and meta-analysis of observational studies. PLoS One. 2013; 8:e55030.

4. Barclay AW, Petocz P, McMillan-Price J, Flood VM, Prvan T, Mitchell P, Brand-Miller JC. Glycemic index, glycemic load, and chronic disease risk--a meta-analysis of observational studies. Am J Clin Nutr. 2008; 87:627–637.

5. Inaba Y, Chen JA, Bergmann SR. Prediction of future cardiovascular outcomes by flow-mediated vasodilatation of brachial artery: a metaanalysis. Int J Cardiovasc Imaging. 2010; 26:631–640.

6. Thijssen DH, Black MA, Pyke KE, Padilla J, Atkinson G, Harris RA, Parker B, Widlansky ME, Tschakovsky ME, Green DJ. Assessment of flow-mediated dilation in humans: a methodological and physiological guideline. Am J Physiol Heart Circ Physiol. 2011; 300:H2–H12.

7. Corretti MC, Anderson TJ, Benjamin EJ, Celermajer D, Charbonneau F, Creager MA, Deanfield J, Drexler H, Gerhard-Herman M, Herrington D, Vallance P, Vita J, Vogel R. International Brachial Artery Reactivity Task Force. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol. 2002; 39:257–265.

8. Roberts AC, Porter KE. Cellular and molecular mechanisms of endothelial dysfunction in diabetes. Diab Vasc Dis Res. 2013; 10:472–482.

9. Vistoli G, De Maddis D, Cipak A, Zarkovic N, Carini M, Aldini G. Advanced glycoxidation and lipoxidation end products (AGEs and ALEs): an overview of their mechanisms of formation. Free Radic Res. 2013; 47:Suppl 1. 3–27.

10. Das Evcimen N, King GL. The role of protein kinase C activation and the vascular complications of diabetes. Pharmacol Res. 2007; 55:498–510.

11. Oates PJ. Aldose reductase, still a compelling target for diabetic neuropathy. Curr Drug Targets. 2008; 9:14–36.

12. Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 2005; 54:1615–1625.
13. Dokken BB. The pathophysiology of cardiovascular disease and diabetes: beyond blood pressure and lipids. Diabetes Spectr. 2008; 21:160–165.

14. El-Osta A, Brasacchio D, Yao D, Pocai A, Jones PL, Roeder RG, Cooper ME, Brownlee M. Transient high glucose causes persistent epigenetic changes and altered gene expression during subsequent normoglycemia. J Exp Med. 2008; 205:2409–2417.

15. Ceriello A, Bortolotti N, Crescentini A, Motz E, Lizzio S, Russo A, Ezsol Z, Tonutti L, Taboga C. Antioxidant defences are reduced during the oral glucose tolerance test in normal and non-insulin-dependent diabetic subjects. Eur J Clin Invest. 1998; 28:329–333.

16. Ceriello A, Assaloni R, Da Ros R, Maier A, Piconi L, Quagliaro L, Esposito K, Giugliano D. Effect of atorvastatin and irbesartan, alone and in combination, on postprandial endothelial dysfunction, oxidative stress, and inflammation in type 2 diabetic patients. Circulation. 2005; 111:2518–2524.

17. Watanabe K, Oba K, Suzuki T, Ouchi M, Suzuki K, Futami-Suda S, Sekimizu K, Yamamoto N, Nakano H. Oral glucose loading attenuates endothelial function in normal individual. Eur J Clin Invest. 2011; 41:465–473.

18. Mah E, Noh SK, Ballard KD, Matos ME, Volek JS, Bruno RS. Postprandial hyperglycemia impairs vascular endothelial function in healthy men by inducing lipid peroxidation and increasing asymmetric dimethylarginine:arginine. J Nutr. 2011; 141:1961–1968.

19. Title LM, Cummings PM, Giddens K, Nassar BA. Oral glucose loading acutely attenuates endothelium-dependent vasodilation in healthy adults without diabetes: an effect prevented by vitamins C and E. J Am Coll Cardiol. 2000; 36:2185–2191.

20. Ceriello A, Taboga C, Tonutti L, Quagliaro L, Piconi L, Bais B, Da Ros R, Motz E. Evidence for an independent and cumulative effect of postprandial hypertriglyceridemia and hyperglycemia on endothelial dysfunction and oxidative stress generation: effects of short- and long-term simvastatin treatment. Circulation. 2002; 106:1211–1218.

21. Xiang GD, Sun HL, Zhao LS, Hou J, Yue L, Xu L. The antioxidant alphalipoic acid improves endothelial dysfunction induced by acute hyperglycaemia during OGTT in impaired glucose tolerance. Clin Endocrinol (Oxf). 2008; 68:716–723.

22. Lee IK, Kim HS, Bae JH. Endothelial dysfunction: its relationship with acute hyperglycaemia and hyperlipidemia. Int J Clin Pract Suppl. 2002; 59–64.
24. Nordmann AJ, Nordmann A, Briel M, Keller U, Yancy WS Jr, Brehm BJ, Bucher HC. Effects of low-carbohydrate vs low-fat diets on weight loss and cardiovascular risk factors: a meta-analysis of randomized controlled trials. Arch Intern Med. 2006; 166:285–293.

25. Schwingshackl L, Hoffmann G. Comparison of effects of long-term low-fat vs high-fat diets on blood lipid levels in overweight or obese patients: a systematic review and meta-analysis. J Acad Nutr Diet. 2013; 113:1640–1661.

26. Keogh JB, Brinkworth GD, Noakes M, Belobrajdic DP, Buckley JD, Clifton PM. Effects of weight loss from a very-low-carbohydrate diet on endothelial function and markers of cardiovascular disease risk in subjects with abdominal obesity. Am J Clin Nutr. 2008; 87:567–576.

27. Merino J, Kones R, Ferré R, Plana N, Girona J, Aragonés G, Ibarretxe D, Heras M, Masana L. Negative effect of a low-carbohydrate, high-protein, high-fat diet on small peripheral artery reactivity in patients with increased cardiovascular risk. Br J Nutr. 2013; 109:1241–1247.

28. Buscemi S, Cosentino L, Rosafio G, Morgana M, Mattina A, Sprini D, Verga S, Rini GB. Effects of hypocaloric diets with different glycemic indexes on endothelial function and glycemic variability in overweight and in obese adult patients at increased cardiovascular risk. Clin Nutr. 2013; 32:346–352.

29. Keogh JB, Brinkworth GD, Clifton PM. Effects of weight loss on a low-carbohydrate diet on flow-mediated dilatation, adhesion molecules and adiponectin. Br J Nutr. 2007; 98:852–859.

30. Varady KA, Bhutani S, Klempel MC, Phillips SA. Improvements in vascular health by a low-fat diet, but not a high-fat diet, are mediated by changes in adipocyte biology. Nutr J. 2011; 10:8.

31. Wycherley TP, Brinkworth GD, Keogh JB, Noakes M, Buckley JD, Clifton PM. Long-term effects of weight loss with a very low carbohydrate and low fat diet on vascular function in overweight and obese patients. J Intern Med. 2010; 267:452–461.

32. Schwingshackl L, Hoffmann G. Low-carbohydrate diets impair flow-mediated dilatation: evidence from a systematic review and metaanalysis. Br J Nutr. 2013; 110:969–970.

33. Halton TL, Willett WC, Liu S, Manson JE, Albert CM, Rexrode K, Hu FB. Low-carbohydrate-diet score and the risk of coronary heart disease in women. N Engl J Med. 2006; 355:1991–2002.

34. McCullough ML, Feskanich D, Stampfer MJ, Giovannucci EL, Rimm EB, Hu FB, Spiegelman D, Hunter DJ, Colditz GA, Willett WC. Diet quality and major chronic disease risk in men and women: moving toward improved dietary guidance. Am J Clin Nutr. 2002; 76:1261–1271.

35. Keogh JB, Grieger JA, Noakes M, Clifton PM. Flow-mediated dilatation is impaired by a high-saturated fat diet but not by a high-carbohydrate diet. Arterioscler Thromb Vasc Biol. 2005; 25:1274–1279.

36. Jenkins DJ, Wolever TM, Taylor RH, Barker H, Fielden H, Baldwin JM, Bowling AC, Newman HC, Jenkins AL, Goff DV. Glycemic index of foods: a physiological basis for carbohydrate exchange. Am J Clin Nutr. 1981; 34:362–366.

37. Wolever TM, Mehling C. High-carbohydrate-low-glycaemic index dietary advice improves glucose disposition index in subjects with impaired glucose tolerance. Br J Nutr. 2002; 87:477–487.

38. McMillan-Price J, Petocz P, Atkinson F, O'Neill K, Samman S, Steinbeck K, Caterson I, Brand-Miller J. Comparison of 4 diets of varying glycemic load on weight loss and cardiovascular risk reduction in overweight and obese young adults: a randomized controlled trial. Arch Intern Med. 2006; 166:1466–1475.

39. Wolever TM, Jenkins DJ, Ocana AM, Rao VA, Collier GR. Second-meal effect: low-glycemic-index foods eaten at dinner improve subsequent breakfast glycemic response. Am J Clin Nutr. 1988; 48:1041–1047.

40. Recio-Rodriguez JI, Gomez-Marcos MA, Patino-Alonso MC, Rodrigo-De Pablo E, Cabrejas-Sánchez A, Arietaleanizbeaskoa MS, Repiso-Gento I, Gonzalez-Viejo N, Maderuelo-Fernandez JA, Agudo-Conde C, Garcia-Ortiz L. EVIDENT Group. Glycemic index, glycemic load, and pulse wave reflection in adults. Nutr Metab Cardiovasc Dis. 2015; 25:68–74.

41. Kim DH, Braam B. Assessment of arterial stiffness using applanation tonometry. Can J Physiol Pharmacol. 2013; 91:999–1008.

42. Song BG, Park JB, Cho SJ, Lee SY, Kim JH, Choi SM, Park JH, Park YH, Choi JO, Lee SC, Park SW. Pulse wave velocity is more closely associated with cardiovascular risk than augmentation index in the relatively low-risk population. Heart Vessels. 2009; 24:413–418.

43. Lavi T, Karasik A, Koren-Morag N, Kanety H, Feinberg MS, Shechter M. The acute effect of various glycemic index dietary carbohydrates on endothelial function in nondiabetic overweight and obese subjects. J Am Coll Cardiol. 2009; 53:2283–2287.

44. Buscemi S, Cosentino L, Rosafio G, Morgana M, Mattina A, Sprini D, Verga S, Rini GB. Effects of hypocaloric diets with different glycemic indexes on endothelial function and glycemic variability in overweight and in obese adult patients at increased cardiovascular risk. Clin Nutr. 2013; 32:346–352.

45. Adolphe JL, Drew MD, Huang Q, Silver TI, Weber LP. Postprandial impairment of flow-mediated dilation and elevated methylglyoxal after simple but not complex carbohydrate consumption in dogs. Nutr Res. 2012; 32:278–284.

46. Liu S, Willett WC, Stampfer MJ, Hu FB, Franz M, Sampson L, Hennekens CH, Manson JE. A prospective study of dietary glycemic load, carbohydrate intake, and risk of coronary heart disease in US women. Am J Clin Nutr. 2000; 71:1455–1461.

47. Mirrahimi A, de Souza RJ, Chiavaroli L, Sievenpiper JL, Beyene J, Hanley AJ, Augustin LS, Kendall CW, Jenkins DJ. Associations of glycemic index and load with coronary heart disease events: a systematic review and meta-analysis of prospective cohorts. J Am Heart Assoc. 2012; 1:e000752.

48. Dong JY, Zhang YH, Wang P, Qin LQ. Meta-analysis of dietary glycemic load and glycemic index in relation to risk of coronary heart disease. Am J Cardiol. 2012; 109:1608–1613.

49. Jenkins DJ, Kendall C, Vuksan V, Jones P, Lamarche B. Canola enriched mediterranean type weight loss diet in type 2 diabetes [Internet]. 2014. cited 2015 Mar 5. Available from https://clinicaltrials.gov/ct2/show/study/NCT02245399.
50. Sacks FM, Carey VJ, Anderson CA, Miller ER 3rd, Copeland T, Charleston J, Harshfield BJ, Laranjo N, McCarron P, Swain J, White K, Yee K, Appel LJ. Effects of high vs low glycemic index of dietary carbohydrate on cardiovascular disease risk factors and insulin sensitivity: the OmniCarb randomized clinical trial. JAMA. 2014; 312:2531–2541.

51. Cozma AI, Sievenpiper JL, de Souza RJ, Chiavaroli L, Ha V, Wang DD, Mirrahimi A, Yu ME, Carleton AJ, Di Buono M, Jenkins AL, Leiter LA, Wolever TM, Beyene J, Kendall CW, Jenkins DJ. Effect of fructose on glycemic control in diabetes: a systematic review and meta-analysis of controlled feeding trials. Diabetes Care. 2012; 35:1611–1620.
52. Jayalath VH, Sievenpiper JL, de Souza RJ, Ha V, Mirrahimi A, Santaren ID, Blanco Mejia S, Di Buono M, Jenkins AL, Leiter LA, Wolever TM, Beyene J, Kendall CW, Jenkins DJ. Total fructose intake and risk of hypertension: a systematic review and meta-analysis of prospective cohorts. J Am Coll Nutr. 2014; 33:328–339.

53. Sievenpiper JL, de Souza RJ, Mirrahimi A, Yu ME, Carleton AJ, Beyene J, Chiavaroli L, Di Buono M, Jenkins AL, Leiter LA, Wolever TM, Kendall CW, Jenkins DJ. Effect of fructose on body weight in controlled feeding trials: a systematic review and meta-analysis. Ann Intern Med. 2012; 156:291–304.

54. David Wang D, Sievenpiper JL, de Souza RJ, Cozma AI, Chiavaroli L, Ha V, Mirrahimi A, Carleton AJ, Di Buono M, Jenkins AL, Leiter LA, Wolever TM, Beyene J, Kendall CW, Jenkins DJ. Effect of fructose on postprandial triglycerides: a systematic review and meta-analysis of controlled feeding trials. Atherosclerosis. 2014; 232:125–133.

55. Memon MQ, Simpson EJ, Macdonald IA. Effect of fructose and sucralose on flow-mediated vasodilatation in healthy, white European males. J Pak Med Assoc. 2014; 64:743–747.
56. Jia G, Aroor AR, Whaley-Connell AT, Sowers JR. Fructose and uric acid: is there a role in endothelial function? Curr Hypertens Rep. 2014; 16:434.

57. Phillips SA, Jurva JW, Syed AQ, Syed AQ, Kulinski JP, Pleuss J, Hoffmann RG, Gutterman DD. Benefit of low-fat over low-carbohydrate diet on endothelial health in obesity. Hypertension. 2008; 51:376–382.

58. Buscemi S, Verga S, Tranchina MR, Cottone S, Cerasola G. Effects of hypocaloric very-low-carbohydrate diet vs. Mediterranean diet on endothelial function in obese women*. Eur J Clin Invest. 2009; 39:339–347.

59. Mohler ER 3rd, Sibley AA, Stein R, Davila-Roman V, Wyatt H, Badellino K, Rader DJ, Klein S, Foster GD. Endothelial function and weight loss: comparison of low-carbohydrate and low-fat diets. Obesity (Silver Spring). 2013; 21:504–509.

60. Ruth MR, Port AM, Shah M, Bourland AC, Istfan NW, Nelson KP, Gokce N, Apovian CM. Consuming a hypocaloric high fat low carbohydrate diet for 12 weeks lowers C-reactive protein, and raises serum adiponectin and high density lipoprotein-cholesterol in obese subjects. Metabolism. 2013; 62:1779–1787.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download