Abstract
For dural arteriovenous fistula (DAVF), when the usual endovascular or neurosurgical approaches are difficult to treat, multi-modal treatment can be helpful. We present a case of a 71-year-old woman with DAVF, who presented with an intracerebral haemorrhage. Digital subtraction angiography revealed a DAVF of the transverse sinus, with cortical venous reflux. Transvenous and transarterial approaches for coil embolization failed. In the operating room, a small craniotomy was performed, and coil embolization was done under fluoroscopy. Transcranial venous embolization might be a useful method to occlude DAVF in a case that is difficult to access by usual surgical or endovascular approaches.
Dural arteriovenous fistula (DAVF) is an abnormal shunt inside the dura, accounting for 10–15% of intracranial arteriovenous malformations.9) DAVF is a rare aetiology of spontaneous intracerebral haemorrhage (ICH),8) but the annual bleeding rate of DAVF is estimated at approximately 1.8–20%.7) If the lesion has cortical venous reflux (CVR) or was located at the tentorium, the bleeding risk is higher.1)
DAVF can be treated with endovascular embolization, surgery or stereotactic radiosurgery alone but sometimes a combined method is necessary. We present our experience with DAVF with ICH and CVR treated with venous embolization, using a transcranial approach, a combination of surgical and endovascular methods under fluoroscopic guidance.
A 71-year-old woman was admitted to our hospital with global aphasia. She had no notable past medical history. Laboratory test showed no evidence of coagulopathy. An initial brain computed tomography (CT) showed an ICH about 15 mL on the left parieto-occipital area (Fig. 1). Digital subtraction angiography (DSA) showed a DAVF of the transverse sinus (TS) fed by multiple arteries arising from the left middle meningeal artery and occipital artery. The fistula drained through the right TS, sigmoid sinus (SS), and cortical veins. CVR was seen (Borden classification type III, Cognard classification type IV) and the left TS and SS were not visible (Fig. 2). As the patient was at high risk of a re-bleed because of the CVR and acute haemorrhage, early treatment for DAVF was necessary.
Under local anaesthesia, a transvenous approach through the superior sagittal sinus and left TS was attempted but failed due to tortuous veins. Consequently, a transarterial embolization via the left middle meningeal artery and left occipital artery endeavoured. However, access through the occipital artery failed. Through the middle meningeal artery, a microcatheter reached the DAVF but the coil formed a frame outside the DAVF, so it unsuccessful.
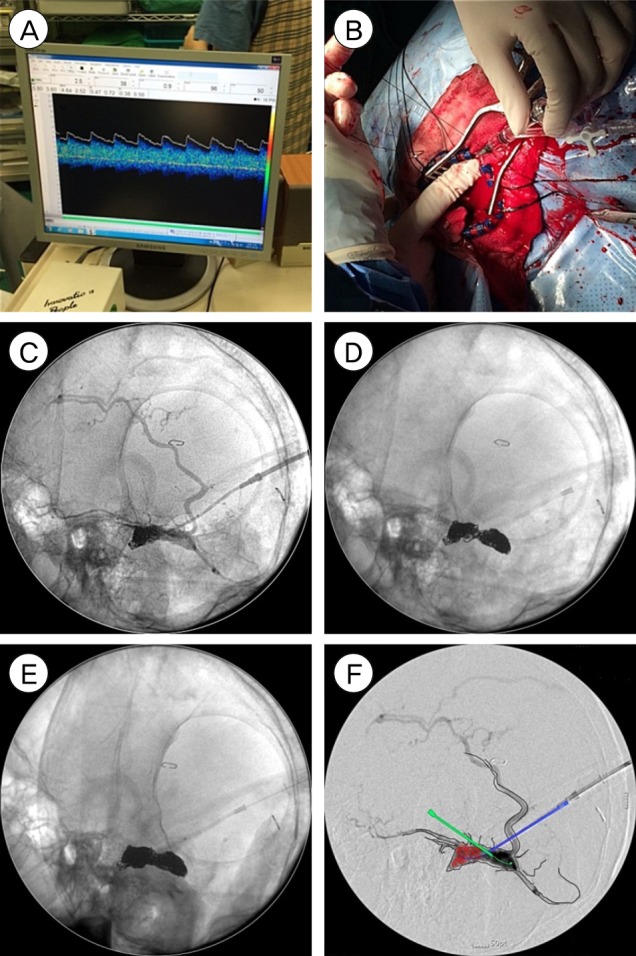
Finally, after moving to the operating room and under general anaesthesia, a retromastoid approach, using a small craniotomy (about 2 × 3 cm) was performed, around the TS SS junction. We identified the location of the sinus by transcranial Doppler (TCD), and arterial pulsation was observed in the isolated left TS at TCD (Fig. 3A). The sinus was punctured with a 16-gauge angiocath needle. Through the angiocath needle, a microcatheter was advanced to the sinus (Fig. 3B).
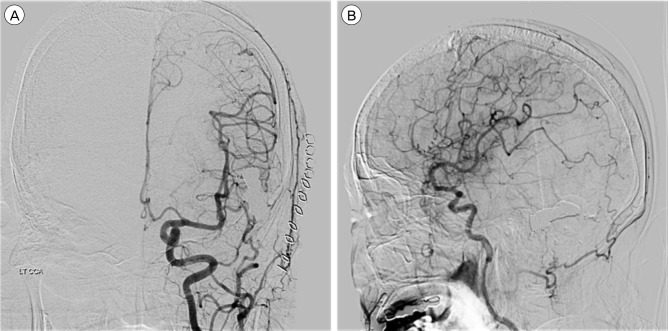
Once the tip of the microcatheter was located within the sinus, fibered coils were inserted, under fluoroscopy, in the operating room (Fig. 3C). The coil mass leaned to one side and, therefore, we punctured the sinus at the opposite side with another angiocath needle (Fig. 3D). We deposited additional fibered coils. Fifty-seven fibered coils were used (Fig. 3E). The left common carotid angiography through the femoral artery showed successful obliteration of the DAVF. To avoid DAVF recurrence, the bone defect was covered with artificial bone made from bone cement, and dura mater tag-up suture was performed.
A week later, CT scans showed haemorrhage resolution without increasing size, and the DSA showed no evidence of partial recanalization (Fig. 4). Global aphasia was slightly improved, and the patient underwent rehabilitation therapy.
DAVFs are arteriovenous shunts from a dural arterial supply to a dural venous drainage channel.4) Many studies show an association between DAVF and venous thrombosis. Whatever the inciting events, the final common path was arteriovenous shunting and secondary venous hypertension. Nonetheless, this strategy can lead to leptomeningeal retrograde drainage and predispose these channels to become varicose and potentially rupture.6) When DAVF is accompanied by CVR, the risk of aggressive symptoms, such as haemorrhage and mortality, is increased; an annual ICH risk of 8.1–19.2% and an annual mortality of 10.4–19.2%.10)
About 20% of patients with a DAVF present with ICH.7) The re-haemorrhage rate is estimated to be up to 35% in the first 2 weeks following an initial haemorrhage3) and, in other studies, as high as 43% in the first few days.2) When DAVF re-bled, 20% of the patients suffered an unfavourable course including death.3) Thus, DAVF with acute haemorrhage requires prompt treatment.
Treatment of DAVF with CVR is aimed at occlusion of the venous drainage or all arterial supply. In many cases, DAVF is treatable with a single modality. However, a single approach might sometimes be difficult and, in such instances, treatment typically involves a combination of modalities. Combined approaches, such as venous embolization under transcranial approach, are commonly done4)5) for DAVF that are difficult to treat by the usual transvenous or transarterial approach alone. The advantage of venous embolization with the transcranial approach is minimising risk and improving outcome. Due to the short distance to the fistula, fistula access is simple, and craniotomy allows multiple needle punctures. Furthermore, compared to surgical resection, blood loss is lower. Despite these advantages, there can be a problem with the equipment and patient transfer if the operating room and angio-room are separate.
Our case was difficult to treat by a single modality. First, we tried an endovascular approach in the angio-room. When that failed, we moved to the operating room, did a craniotomy, and punctured the sinus for coil embolization. Embolization performed in the operating room with fluoroscopy may lead to partial occlusion because the resolution of fluoroscopy is worse than the angiogram. In contrast, as the operating room is located on the third floor but the angio-room on the first basement level, it is challenging to transfer the patient to the angio-room in general anaesthesia, while leaving multiple devices in the body and brain. As fluoroscopy is highly available, and accessible without moving the patient, we selected coil embolization in the operating room using fluoroscopy. In this way, the lesion was completely obliterated.
A growing trend is the hybrid operating room, obviating the need to move the patient, reducing both the risk associated with movement and the time under general anaesthesia. There is an increasing preference for non-invasive treatment. In determining the treatment policy, the transcranial venous approach, in a case like this, might be able to reduce bleeding risk and perioperative risk. In order to reduce the patient's perioperative risk, a hybrid operating room might be helpful in difficult access cases, with the development of instruments and endovascular treatment methods.
DAVFs accompanied by ICH and CVR demand urgent treatment, due to re-bleeding risk. When DAVF is challenging to treat by a single modality, combined modalities can be beneficial. A venous embolization with transcranial approach has many advantages, including simple fistula access, allows multiple needle punctures and has low blood loss. Fluoroscopy-guided coil embolization has inherent risks of incomplete obliteration. Hence, to reduce perioperative risk related with combined modalities, along with endovascular treatment methods and instrumental developments, a hybrid operating room might be effective.
References
1. Awad IA, Little JR, Akarawi WP, Ahl J. Intracranial dural arteriovenous malformations: factors predisposing to an aggressive neurological course. J Neurosurg. 1990; 6. 72(6):839–850. PMID: 2140125.

2. Byun JS, Hwang SN, Park SW, Nam TK. Dural arteriovenous fistula of jugular foramen with subarachnoid hemorrhage : selective transarterial embolization. J Korean Neurosurg Soc. 2009; 3. 45(3):199–202. PMID: 19352487.

3. Duffau H, Lopes M, Janosevic V, Sichez JP, Faillot T, Capelle L, et al. Early rebleeding from intracranial dural arteriovenous fistulas: report of 20 cases and review of the literature. J Neurosurg. 1999; 1. 90(1):78–84. PMID: 10413159.

4. Halbach VV, Higashida RT, Hieshima GB, Mehringer CM, Hardin CW. Transvenous embolization of dural fistulas involving the transverse and sigmoid sinuses. AJNR Am J Neuroradiol. 1989; Mar-Apr. 10(2):385–392. PMID: 2494858.
5. Houdart E, Saint-Maurice JP, Chapot R, Ditchfield A, Blanquet A, Lot G, et al. Transcranial approach for venous embolization of dural arteriovenous fistulas. J Neurosurg. 2002; 8. 97(2):280–286. PMID: 12186454.

6. Houser OW, Campbell JK, Campbell RJ, Sundt TM Jr. Arteriovenous malformation affecting the transverse dural venous sinus--an acquired lesion. Mayo Clin Proc. 1979; 10. 54(10):651–661. PMID: 480990.
7. Iwama T, Hashimoto N, Takagi Y, Tanaka M, Yamamoto S, Nishi S, et al. Hemodynamic and metabolic disturbances in patients with intracranial dural arteriovenous fistulas: positron emission tomography evaluation before and after treatment. J Neurosurg. 1997; 5. 86(5):806–811. PMID: 9126896.

8. Jolink WM, van Dijk JM, van Asch CJ, de Kort GA, Algra A, Groen RJ, et al. Outcome after intracranial haemorrhage from dural arteriovenous fistulae; a systematic review and case-series. J Neurol. 2015; 12. 262(12):2678–2683. PMID: 26410748.

9. Newton TH, Cronqvist S. Involvement of dural arteries in intracranial arteriovenous malformations. Radiology. 1969; 11. 93(5):1071–1078. PMID: 5350675.

10. van Dijk JM, terBrugge KG, Willinsky RA, Wallace MC. Clinical course of cranial dural arteriovenous fistulas with long-term persistent cortical venous reflux. Stroke. 2002; 5. 33(5):1233–1236. PMID: 11988596.

Fig. 1
Axial non-contrast brain computed tomography shows an intracerebral haemorrhage on left parieto-occipital area.
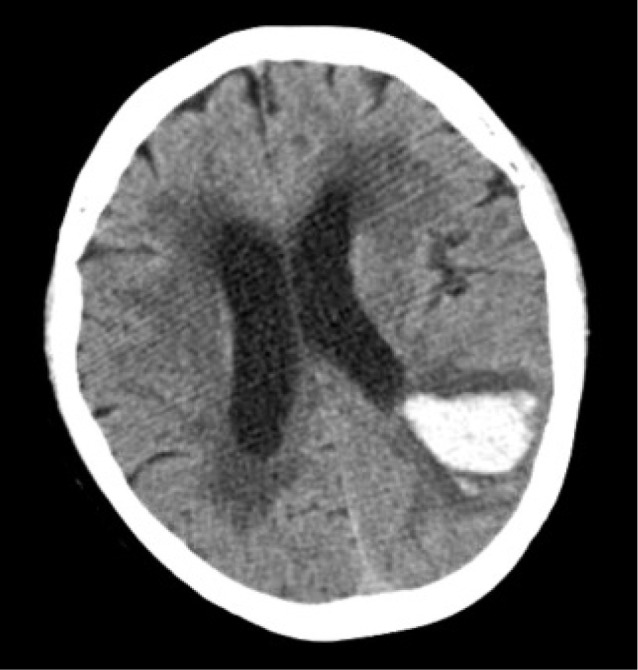
Fig. 2
Digital subtraction angiography, left external carotid artery injection, anteroposterior (A) and lateral (B) views. Angiogram revealing an isolated left transverse sinus and dural arteriovenous fistula fed by left middle meningeal artery and occipital artery with ectatic venous drainage.
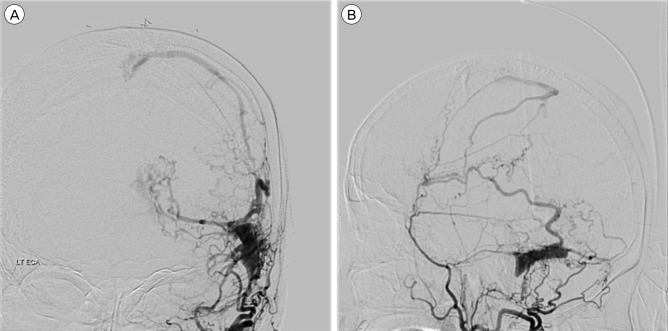
Fig. 3
Illustration of coil embolization under C-arm image guiding after craniotomy. (A) Arterial pulsation was observed in vein of transverse sinus at transcranial Doppler. (B) An angiocath needle was placed in the isolated sinus and preparation for coil embolization was performed through the needle. (C) The isolated sinus was punctured with an angiocath needle and coils were inserted. (D) The sinus was punctured at the opposite side with another angiocath needle and coils were inserted. (E) Coil masses were met from both sides. (F) Sketch demonstrated the isolated sinus packed with coils, two punctured angiocath needles and feeding arteries (left middle meningeal artery and left occipital artery).




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download